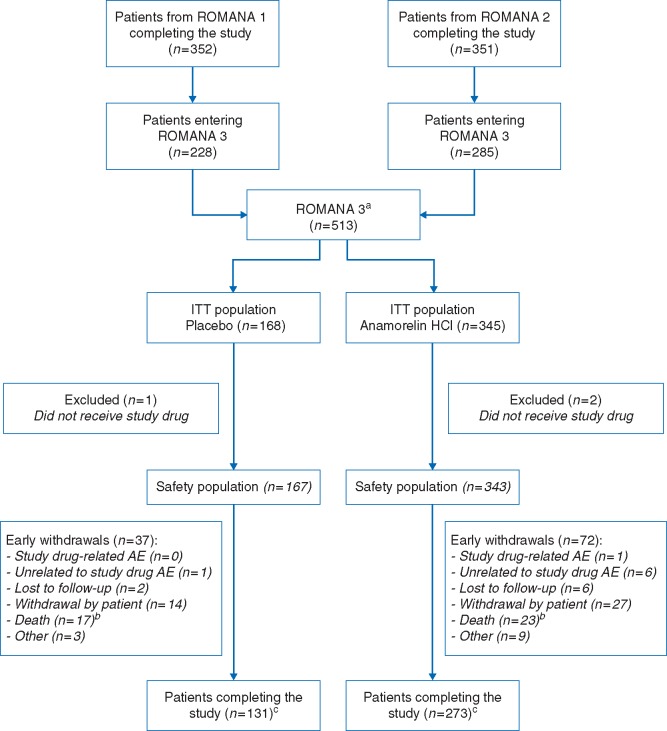
Figure 1.
CONSORT diagram of ROMANA 3. aPatients enrolled in ROMANA 3 stayed on their initial treatment arm (from ROMANA 1 or ROMANA 2). bNo deaths were drug related. cIncluding the patients who did not receive study drug. Patients completing the study are defined as patients finalizing the week 12/day 85 visit. AE, adverse event; ITT, intent-to-treat.