Abstract
The level of proinflammatory markers was assessed in HIV-infected patients that were coinfected with hepatitis C virus (HCV) and had failed to restore the CD4+ T cell counts (immunological nonresponders, INR) during the antiretroviral therapy (ART). Among four patient groups (HIV+HCV− and HIV+HCV+ subjects with the concordant response to ART; HIV+HCV− and HIV+HCV+ subjects that were INR), the greatest systemic inflammation was in the latter group. The maximum difference was between the subjects HIV+HCV−INR and HIV+HCV+ INR: the blood of coinfected patients contained significantly higher concentrations of the IP-10, sCD163, sTNF-RI, and sTNF-RII and of bacterial lipopolysaccharide. Systemic inflammation in HIV/HCV coinfected patients with the discordant response to ART is probably caused by a breach of hepatic barrier for the intestine products.
In HIV-infected patients, antiretroviral therapy (ART) not only suppresses virus replication, but also leads to restoration of the number of T cells CD4+ [1]. Nevertheless, in about 20% of the ART-treated patients, an ability to restore these cells is low, in spite of complete suppression of the viral load [1–5]. In the published reports, these subjects are referred to as immunological non-responders (INR). We have earlier found [6] that, in the HIV-infected INR coinfected with the hepatitis C virus (HCV), hepatic tissue destruction was more severe than in the coinfected HIV/HCV subjects with standard immune response to the therapy (immunological responders, IR). In addition, HCV coinfection is known to promote the development of systemic inflammation in patients with HIV infection [7, 8].
The objective of this study was to assess the degree of systemic inflammation in the ART-treated HIV/HCV patients with impaired recovery of CD4+ T cells.
Seventy nine HIV-infected patients and 20 non-infected volunteers have been examined. HIV-infected patients were divided into the following four groups: group 1, HIV+HCV+, IR (the number of T cells CD4+ > 350/μL); group 2, HIV+HCV+, INR (the number of T cells CD4+ < 350/μL); group 3, HIV+HCV−, IR (the number of T cells CD4+ > 350/μL); group 4, HIV+HCV−, INR, (the number of T cells CD4+ < 350/μL). Before therapy, the level of T cells CD4+ in blood was <200/μL. After ART (for more than two years), HIV replication was completely suppressed in all subjects (<50 copies/mL). The coinfected patients did not receive interferon therapy. The average age of patients in different groups was the following (years): group 1, 35.4 ± 1.6; group 2, 34.5 ± 0.8; group 3, 36.2 ± 1.9; group 4, 35.8 ± 1.4. The average age of the non-infected volunteers was 32.3 years. No statistically significant age-dependent differences were found between the groups. This study was approved by the ethical Committee of Perm Regional Center for Protection against AIDS and Infectious Diseases. An informed written consent has been obtained from the examined subjects. We used ELISA and the kits from R&D Systems (United States) to determine the concentrations of IL-6, IP-10, soluble CD163 (sCD163), and CD14 (sCD14), soluble cell receptors for TNF-α (sTNF-RI and sTNF-RII) and for the intestinal fatty acid-binding protein (I-FABP) in blood plasma. Neopterin was assessed using a kit from IBL International (Germany). The blood content of the bacterial lipopolysaccharide (LPS) was measured by the quantitative LAL test and Uden reagents (Netherlands). The levels of IL-10 were determined using the kits from Vector-Best (Russia). All procedures were performed according to the manufacturer recommendations.
Statistical calculations and plotting were performed using the Statistica 6.0. Significant differences were assessed by the Wilcoxon–Mann–Whitney U test and Student’s t test.
The content of proinflammatory markers in blood of the HIV-infected subjects indicates that systemic inflammation was in all of these patients (Table 1). However, its intensity was not equal in different groups of patients. The maximum level of inflammation was in HIV/HCV coinfected INR. Groups 1 and 2 differed only in the content of sCD163. Hepatitis development in these patients was accompanied by a different extent of the hepatic tissue destruction. Patients of groups 3 and 4 also had no significant differences in the level of proinflammatory markers. Thus, systemic inflammation was not a result of poor immune recovery during ART.
Table 1.
Systemic inflammatory markers in HIV/HCV-coinfected patients with disturbed CD4+ T-cell recovery after ART
| Inflammatory markers | HIV+HCV+ | HIV+HCV+ | HIV+HCV− | HIV+HCV− | Control (no infection) | p* |
|---|---|---|---|---|---|---|
|
| ||||||
| IR | INR | IR | INR | |||
|
| ||||||
| 1 | 2 | 3 | 4 | 5 | ||
|
| ||||||
| IL-6, pg/mL | 1.2 (0.9–1.5) |
1.0 (0.9–1.2) |
0.9 (0.7–1.0) |
0.9 (0.7–1.3) |
0.6 (0.4–0.8) |
р1,5 < 0.001 р2,5 < 0.001 р3,5 < 0.01 |
| IР-10, ng/mL | 0.8 (0.6–0.8) |
1.1 (0.7–1.5) |
0.3 (0.2–0.5) |
0.3 (0.2–0.4) |
0.2 (0.2–0.4) |
р1,5 < 0.001 р2,5 < 0.001 р1,3 < 0.001 р2,4 < 0.001 |
| sCD163, μg/mL | 1.1 (0.7–1.7) |
1.4 (1.1–2.2) |
0.7 (0.5–1.0) |
0.9 (0.6–1.0) |
0.7 (0.5–0.8) |
р1,5 < 0.01 р2,5 < 0.001 р1,2 < 0.05 р1,3 < 0.01 р2,4 < 0.001 |
| Neopterin, nmol/L | 8.4 (7.3–10.5) |
11.0 (8.2–12.8) |
6.4 (5.4–8.7) |
8.9 (6.2–11.3) |
5.1 (4.5–7.7) |
р1,5 < 0.001 р2,5 < 0.001 р1,3 < 0.01 р4,5 < 0.01 |
| sCD14, μg/mL | 1.9 (1.8–2.0) |
1.9 (1.7–2.2) |
1.8 (1.6–2.2) |
1.8 (1.5–2.1) |
1.5 (1.2–1.8) |
р1,5 < 0.01 р2,5 < 0.01 р3,2 < 0.01 р4,5 < 0.05 |
| I-FABP, ng/mL | 1.6 (1.3–2.0) |
1.8 (1.4–2.5) |
1.5 (0.9–2.4) |
1.8 (1.2–2.6) |
1.0 (0.7–1.4) |
р1,5 < 0.01 р2,5 < 0.01 р4,5 < 0.01 |
| TNF-RI, ng/mL | 1.0 (0.8–1.2) |
1.1 (0.9–1.3) |
0.9 (0.6–1.1) |
0.9 (0.7–1.0) |
1.0 (0.8–1.1) |
р2,4 < 0.05 |
| TNF-RII, ng/mL | 2.9 (2.5–3.4) |
3.0 (2.6–3.4) |
2.4 (2.1–3.0) |
2.6 (1.8–2.8) |
2.5 (2.1–3.0) |
р2,4 < 0.05 р2,5 < 0.05 |
| LPS, IE/L | 90.0 (48.7–119.1) |
95.8 (53.2–138.6) |
78.4 (49.5–103.7) |
44.3 (30.5–50.9) |
41.0 (34.4–56.2) |
р1,5 < 0.01 р2,5 < 0.001 р2,4 < 0.001 р3,4 < 0.01 р3,5 < 0.01 |
Medians and (in parentheses) interquartile ranges are presented;
U test.
When compared the HIV/HCV coinfected and HIV monoinfected patients with normal response of CD4+ T cells to the treatment we found an increase in systemic inflammation against the background of hepatitis. The differences were in concentrations of IP-10, sCD163, and neopterin. Comparison of the HIV+HCV+ and HIV+HCV− groups showed difference in the content of IP-10, sCD163, sTNF-RI, sTNF-RII, and LPS. These data suggest that, in the case of the discordant immune response to ART, the HCV-caused systemic inflammation is more active than in the case of the standard immune response to therapy. The differences in the level of LPS in the two groups of INR stand out. Since I-FABP concentration that characterizes intestine permeability was similar in both groups, a conclusion may be drawn that an increase in LPS concentration in blood of the HIV/HCV-coinfected patients having low recovery potential of CD4+ T cells is a result of the hepatic barrier breach. Hence, a severe inflammatory reaction in the coinfected INRs is probably caused by the more intense transport of microbial products into blood.
Among the factors capable of promoting LPS biological effects, sCD14 and IL-10 should be emphasized. Protein sCD14 prevents interaction of the membrane CD14 with LPS and by this way reduces the inflammatory process [9]. In the HIV-infected patients of any of the four groups, the content of free CD14 receptor that binds to LPS was significantly higher than that in volunteers. In different groups of the HIV-infected patients, this index did not differ significantly. The content of IL-10 in blood plasma of the HIV-infected patients was similar to that in blood plasma of the non-infected volunteers (Fig. 1). Nevertheless, statistically significant differences were observed between two groups of the ART-treated patients with disturbed CD4+ T cells recovery: HIV+HCV+ and HIV+HCV−. An increase in the level of IL-10 was characteristic of the coinfected patients. It may be that like sCD14, IL-10 prevents inflammation development in the coinfected patients. Cytokine IL-10 is known to be a factor that attenuates tissue destruction during immune response and inflammation. Its deficiency leads to a severe course of the experimental autoimmune diseases [10, 11]. In viral infections, IL-10 suppresses proinflammatory effects of both innate and adaptive immunity [12].
Fig. 1.
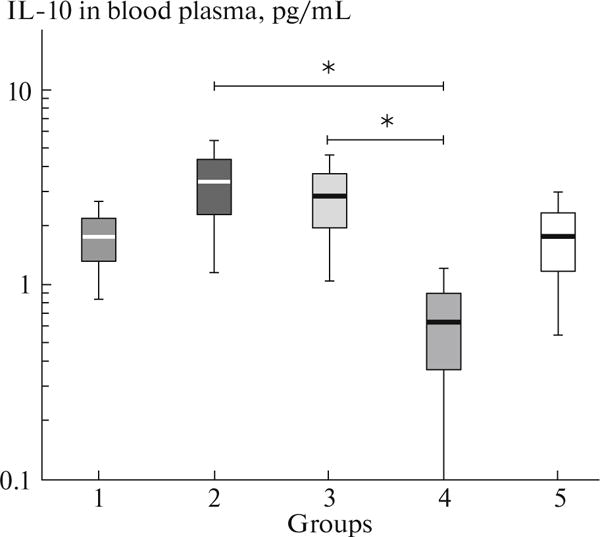
IL-10 concentration in blood plasma of HIV/HCV-coinfected patients with discordant and concordant CD4+ T-cell response to therapy. 1, HIV+HCV+, IR; 2, HIV+HCV+, INR; 3, HIV+HCV−, IR; 4, HIV+HCV−, INR; 5, HIV−HCV−. Arithmetic mean values (horizontal lines within rectangles), errors of mean (rectangles), and 95% confidence intervals (vertical lines) are presented; *p < 0.05 according to t test.
Of interest is that, in the case of discordant response to ART, changes in IL-10 concentrations were not unidirectional in patients with HIV/HCV coinfection and in those with HIV monoinfection. This is most likely to be related to the level of LPS in blood. It has been earlier reported that, in patients with HIV monoinfection who received ART and reached undetectable level of the viral load, IL-10 secretion in vitro by mononuclear cells was positively and statistically significant correlated with an increase in the number of CD4+ T cells in blood [13]. It still remains unclear why the low level of IL-10 leads to immune failure of patients with HIV monoinfection. HIV/HCV-coinfected INR with disturbed intestine and hepatic barriers retain the ability to synthesize effectively this anti-inflammatory cytokine.
Our data suggest that impaired regenerative activity of CD4+ T cells in HIV infection can be formed at different severity of systemic inflammation. How this affects an ability of immunocompetent cells to respond to ART is yet to be established.
Acknowledgments
This study was supported by the Russian Foundation for Basic Research (project no. 17-54-30006) and the National Institute of Allergy and Infection Diseases (grant no. AI 36219).
References
- 1.Autran B, Carcelaint G, Li TS, et al. Immunol Lett. 1999;66(1–3):207–211. doi: 10.1016/s0165-2478(98)00159-x. [DOI] [PubMed] [Google Scholar]
- 2.Piketty C, Castiel P, Belec L, et al. AIDS. 1998;12(7):745–750. doi: 10.1097/00002030-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/670957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massanella M, Negredo E, Clotet B, Blanco J. Expert Rev Clin Immunol. 2013;9(11):1135–1149. doi: 10.1586/1744666X.2013.842897. [DOI] [PubMed] [Google Scholar]
- 5.Autran B, Carcelain G, Li TS, et al. Science. 1997;277(5322):112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 6.Shmagel NG, Shmagel KV, Saidakova EV, et al. Dokl Biochem Biophys. 2015;465:358–360. doi: 10.1134/S1607672915060034. [DOI] [PubMed] [Google Scholar]
- 7.Marchetti G, Cozzi-Lepri A, Tincati C, et al. BMC Infect Dis. 2014;14:79. doi: 10.1186/1471-2334-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shmagel KV, Saidakova EV, Shmagel NG, et al. HIV Med. 2016;17(8):581–589. doi: 10.1111/hiv.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wurfel MM, Hailman E, Wright SD. J Exp Med. 1995;181(5):1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beebe AM, Cua DJ, de Waal Malefyt R. Cytokine Growth Factor Rev. 2002;13(4–5):403–412. doi: 10.1016/s1359-6101(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 11.Hata H, Sakaguchi N, Yoshitomi H, et al. J Clin Invest. 2004;114(4):582–588. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojas JM, Avia M, Martin V, Sevilla NJ. Immunol Res. 2017;2017:6104054. doi: 10.1155/2017/6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villacres MC, Kono N, Mack WJ, et al. J Infect Dis. 2012;206(5):780–789. doi: 10.1093/infdis/jis380. [DOI] [PMC free article] [PubMed] [Google Scholar]


