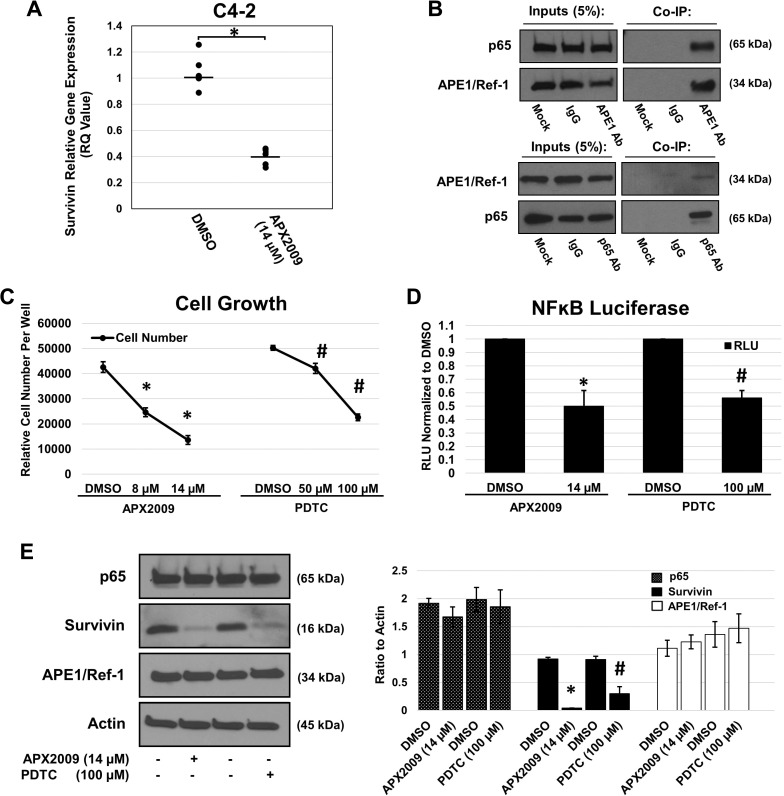
Figure 6. APE1/Ref-1 redox inhibition decreases survivin protein levels via NFκB.
(A) C4-2 cell line was treated with DMSO or APX2009 (14 µM) for 12 hours. RNA was isolated and RT-PCR for survivin was performed with HPRT1 as the reference gene. n = 6, *-denoting p < 0.05 by unpaired Student’s t-test. (B) Immunoblot validation of APE1/Ref-1 and p65 Co-Immunoprecipitation (Co-IP) reactions. A 5% sample of the total input of each reaction (Input) and the total IP reaction (IP) were loaded for each reaction. Beads lacking a conjugated antibody (Mock) and generic IgG (IgG) were used as negative controls for each IP experimental reaction, APE1 antibody (top blots) and p65 (bottom blots). (C) C4-2 cell line was treated with DMSO, APX2009 (8 and 14 µM) or PDTC (50 and 100 µM) for 72 hours and cells were fixed and methylene blue was performed. n = 3, *-denoting p < 0.05 (DMSO vs 8 and 14 µM APX2009) and #-denoting p < 0.05 (DMSO vs. 50 and 100 µM PDTC) as assessed by ANOVA. (D) C4-2 cells were transfected with NFκB–Luc construct and co-transfected with a Renilla vector, pRL-TK. After 16 hours, cells were treated with growth inhibitor IC50 concentrations of APX2009 and PDTC for 24 hours, and Firefly and Renilla luciferase activities were assayed using Renilla luciferase activity for normalization. All transfection experiments were performed in triplicate and repeated 3 times in independent experiments. Data are expressed as Relative Luciferase Units (RLU) normalized to DMSO showing the mean ± SEM. n = 3, *-denoting p < 0.05 (DMSO vs. 14 µM APX2009) and #-denoting p < 0.05 (DMSO vs. 100 µM PDTC) within unpaired Students t-test. (E) C4-2 cell line was treated with APX2009 (14 µM) and NFĸB-selective inhibitor PDTC (100 µM) for 48 hours. Immunoblotting was performed with antibodies for survivin, p65, APE1/Ref-1 and Actin as labeled. Data presented are representative of three determinations with densitometry quantification, n = 33, *-denoting p < 0.05 (DMSO vs. 14 µM APX2009) and #-denoting p < 0.05 (DMSO vs. 100 µM) as assessed by unpaired Student’s t-test.