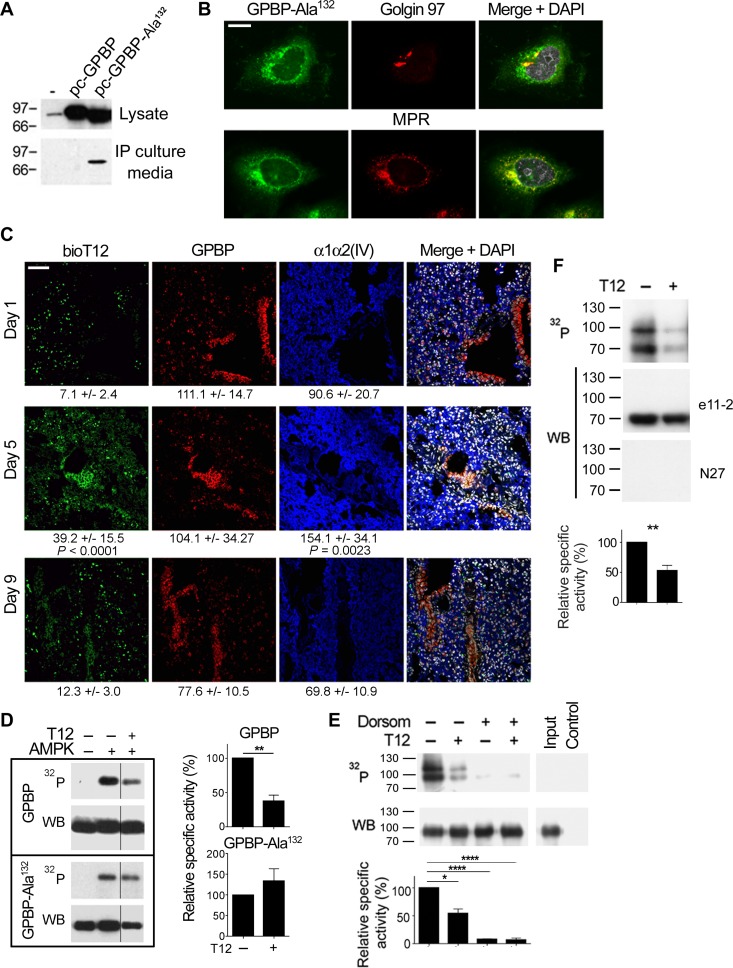
Figure 11. Epithelial cells clear tumor mesenchymal cGPBP levels and generate a previously unrecognized form of GPBP.
(A) HEK 293 cells were transfected with pcDNA3 (–) or the indicated derived constructs coding for FLAG-GPBP or FLAG-GPBP-Ala132. After 36 h, equivalent amounts of the indicated materials were analyzed by WB with anti-GPBP mAb 14 [3]. (B) FLAG-GPBP-Ala132 transfected HEK 293 cells were analyzed by IF-CM using specific antibodies for the indicated polypeptides. FLAG-GPBP-Ala132 was visualized with anti-FLAG. MPR, mannose-6-phosphate receptor (bar = 10 μm). (C) Frozen lung sections from 4T1 mouse-model sacrificed at the indicated days (n = 2) were analyzed by IF-CM to visualize in situ T12 binding (bioT12) and the indicated polypeptides. FI was measured and expressed as in Figure 1. Statistics: 1WA-D comparison respect to day 1. (D) The phosphorylation mixtures containing the indicated FLAG-polypeptides and reagents were analyzed and represented as in previous figures. WB was developed with α-FLAG antibody. (E) A549 cultures were supplemented with BM40-FLAG-GPBP for 6 h in the presence (+) or absence (–) of dorsomorphin. BM40-FLAG-GPBP purified from cellular extracts was phosphorylated with (+) or without (–) T12 and similarly analyzed and represented. Phosphorylation mixtures of BM40-FLAG-GPBP (Input) and anti-FLAG immunoprecipitated material from mock A549 cultures (Control) are also displayed. WB, was developed with N27 antibody. Where indicated in D and E, dorsomorphin and T12 were used at 40 and 50 µM, respectively. Statistics: Student´s t-test, n = 4 (D) n = 2 (E). (F) cGPBP from NSCLC patient’s plasma (0.5 mL) was immunopurified with scFvN26, released using appropriated blocking peptide and subjected to in vitro phosphorylation assay. Resulting phosphorylation mixtures were analyzed and represented as in Figure 5B. Statistics: Student’s t-test, n = 3. Similar results were obtained when pull-down assays were done using scFvN27 and the corresponding blocking peptide, suggesting that cGPBP multimeric structures contained a minority of N27-reactive material that allowed immunoprecipitation and limited detection by EMTEST (<1 ng/mL).