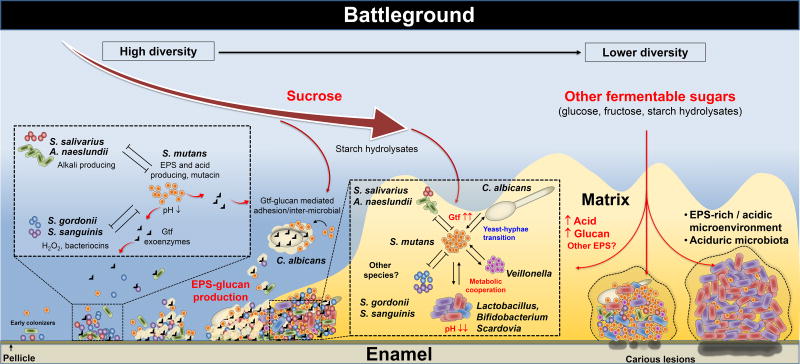
Figure 3. The biofilm battleground: antagonistic, synergistic and mutualistic interactions to build a pathogenic habitat.
The social interactions of the oral microbial community start with early colonizers that can rapidly adhere to the tooth surface, and then co-adhere with other microorganisms. During this process the various species interact physically and metabolically to shape the initial biofilm community. The interactions can be both antagonistic and cooperative, which can dynamically change depending on the host. Certain interactions are beneficial as S. gordonii/S. salivarius and A. naeslundii interfere with caries pathogens such as S. mutans by secreting bacteriocins and hydrogen peroxide as chemical weapons or counter the deleterious effects of acidification by producing alkali. However, the balance between commensals and pathogens can be disrupted by frequent sugar consumption and poor oral care. When sucrose is available, EPS-producing exoenzymes such as Gtfs present in the pellicle and also bound to different microbes (including C. albicans) produce copious amounts of glucans. Furthermore, the Gtfs can also use starch hydrolysates (from α-amylase activity on pellicle and bacterial surfaces) to produce hybrid glucan polymers. The surface-formed glucans provide novel binding sites for adhesion and co-adhesion, which mediates new interspecies interactions and microbial clustering on the tooth surface, while assembling a polymeric matrix that provides protection and mechanical stability, making biofilm recalcitrant to antimicrobials and difficult to remove. Competitive and synergistic interactions continue to develop between the microbes embedded in the biofilm structure. If the biofilm remains on teeth and the consumption of carbohydrate-rich diets persist, the amount of EPS and extent of acidification of the matrix increases. The diffusion-modifying properties of the matrix combined with microbial metabolic activities help create a highly acidic and increasingly anaerobic (hypoxic) niche. Such conditions elicit biochemical, ecological, and structural changes that favor the survival and dominance of highly acid-stress tolerant organisms that can synergize with each other. The microbial diversity can further reduce in favor of an aciduric microbiota, help maintaining an acidified microenvironment. The low-pH condition at tooth-biofilm interface promotes demineralization of the enamel leading to the onset and progression of dental caries. This model may explain the rapid accumulation of cariogenic plaque in the presence of sucrose (and other fermentable sugars) in the diet, even if the initial population of pathogens such as S. mutans is numerically low.