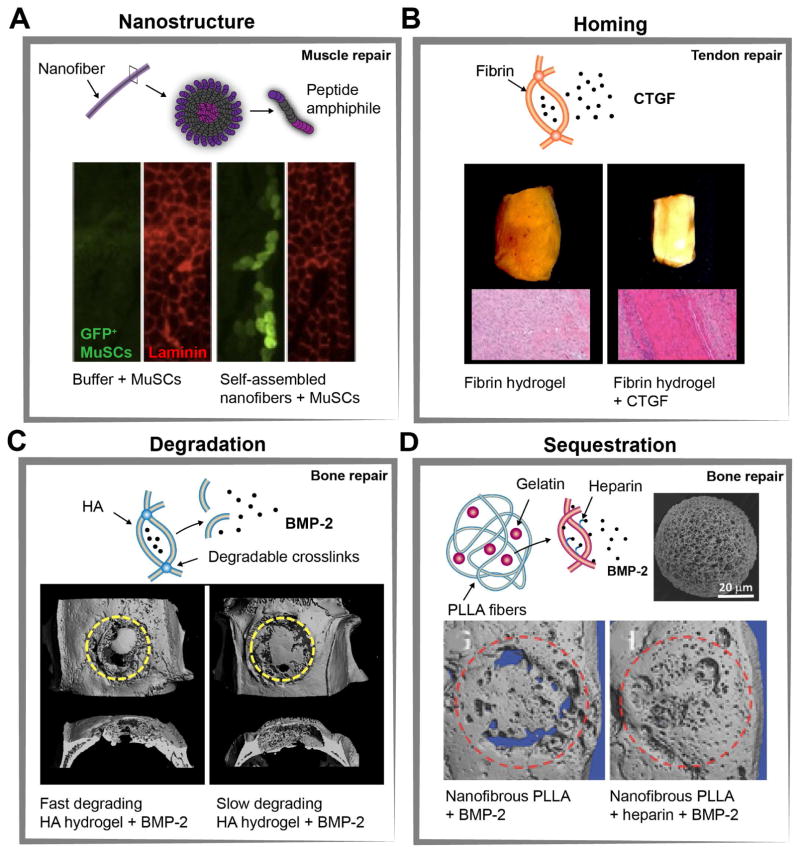
Figure 3. Examples of biomaterials engineered to recruit and control endogenous stem and stromal cell behavior in vivo (A).
Implantation of muscle satellite cells (MuSCs) in self-assembled nanofibers enhanced donor cell mediated repair of myofibers. Representative immunostaining of muscle tissue sections 5 weeks after implantation shows enhanced engraftment of GFP+ MuSCs compared with cells injected in buffer only. (B) Connective tissue growth factor (CTGF) released from fibrin hydrogels improved repair of transected rat patellar tendons. Gross images and representative histological images 4 weeks after implantation showed dense alignment of collagen fibers for fibrin gels with CTGF by stimulating proliferation and differentiation of endogenous tendon progenitor cells. (C) Bone-morphogenetic protein (BMP-2) incorporated into matrix metalloproteinases (MMP)-degradable hyaluronic acid (HA) hydrogels sustained release of BMP-2 through cell-mediated hydrogel degradation. Representative μ-CT images of rat calvarial defects 6 weeks after implantation demonstrate increased bone volume for faster degrading HA-hydrogels. (D) New bone tissue formation can also be increased through implantation of nanofibrous poly(l-lactic acid) (PLLA) microspheres that contain gelatin-heparin/BMP-2 microspheres (scanning electron microscope (SEM) image of a typical nanofibrous PLLA microsphere). Representative μ-CT images of rat calvarial defects 6 weeks after implantation show improved bone regeneration for PLLA microspheres with heparin-conjugated gelatin due to sustained release of BMP-2. Figures are adapted with permission from the following: (A) (Sleep et al., 2017) (B) American Society For Clinical Investigation Ref: (Lee et al., 2015). (C) Elsevier Ref: (Holloway et al., 2014). (D) Wiley Ref: (Ma et al., 2015).