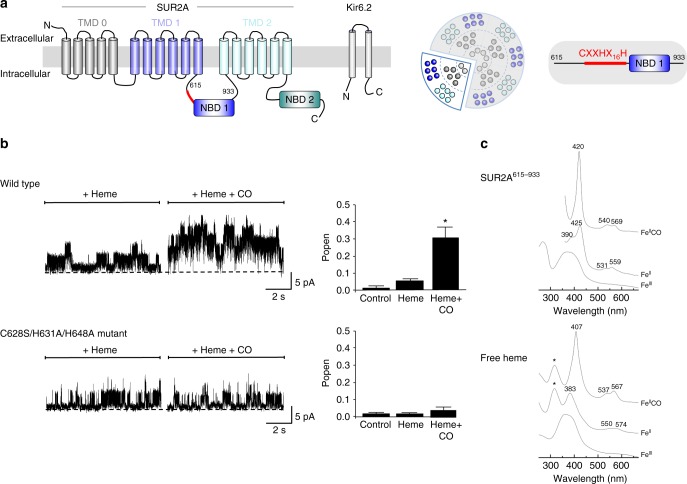
Fig. 1.
Heme is required for CO-dependent increase in channel activity. a Schematic of the KATP channel subunit and protein structure. There are four sulphonylurea receptor (SUR) subunits which are members of the ATP-binding cassette (ABC) transporter subfamily c (SUR2A in this work), and a further four pore-lining subunits of the inward rectifier family (Kir6.2, shown on left). These assemble to form an octameric channel structure (shown in the middle). Red indicates the region of the SUR2A subunit containing the CXXHX16H heme binding motif adjoining the nucleotide-binding domain (NBD1). On the far right is shown schematically the S615-L933 region of rat SUR2A containing NDB1 and the CXXHX16H heme binding motif. b Mutation of residues in the heme binding region or SUR2A abolishes the heme-dependent CO increase in KATP channel open probability. (Top) Inside–out single channel analysis of the effects of heme and heme/CO on wild type KATP channel activity and a summary showing the mean open probability changes in response to heme and CO (n = 6). (Bottom) Effects of mutation of the heme binding site, in the triple C628S/H631A/H648A mutant of SUR2A, on the responses of single channel currents and mean open probability to heme and CO (n = 6, *P < 0.05, paired T test). c Absorption spectra of SUR2A615–933 (top three spectra) and free heme (bottom three spectra) in the ferric (FeIII), ferrous (FeII) and ferrous CO-bound (FeII-CO) forms. The ferric form of free heme is characterized by a broad Soret band centered at 377 nm53, which probably arises from different heme conformations; reduction of free heme with dithionite leads to a red-shifted Soret band at 383 nm which is attributed to a 5-coordinate high spin species and closely resembles that of dithionite-reduced hemin in 0.5% sodium dodecyl sulphate54 (λmax = 395, 405, 425 (weak), 439 (weak) nm). Addition of CO to reduced heme results in the formation of a complex with a Soret band at 407 nm and Q-bands at 537 and 657 nm55