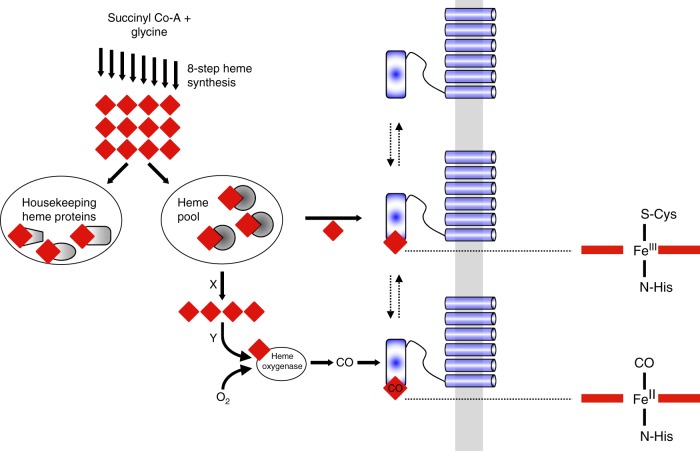
Fig. 4.
A molecular basis for CO-dependent activation of KATP channels. Figure (left) shows the connections that might exist between heme (red diamond) and CO. The 8-step biosynthesis of heme regulates the overall supply. Of the total heme concentration, some is committed to the heme proteins essential for the survival of the cell (left oval). The remainder is thought to exist in a ‘pool’ (right oval), probably bound to unknown chaperone proteins. Heme in this pool is available for regulatory control, including binding to ion channel proteins (blue) bound at the membrane (grey). Partner proteins (X, Y) are envisaged as important for heme transport and/or for reduction of the heme to the ferrous (FeII) state (prior to binding of gaseous ligands). Formation of CO from the catalytic degradation of heme by the O2-dependent heme oxygenase enzyme is envisaged to also interact with heme bound to channel proteins (see text for details). On the far right is shown the proposed ligation in the heme-SUR2A615–933 and heme-CO-SUR2A615–933 complexes. The Cys/His ligation may be retained in the ferrous form, but FeII-thiolate bonds are weak and replacement by a second (protein) ligand is also possible37, 56. Note that the activating effects of CO on the channel are not observed in the absence of heme or when the heme binding site on SUR2A is removed