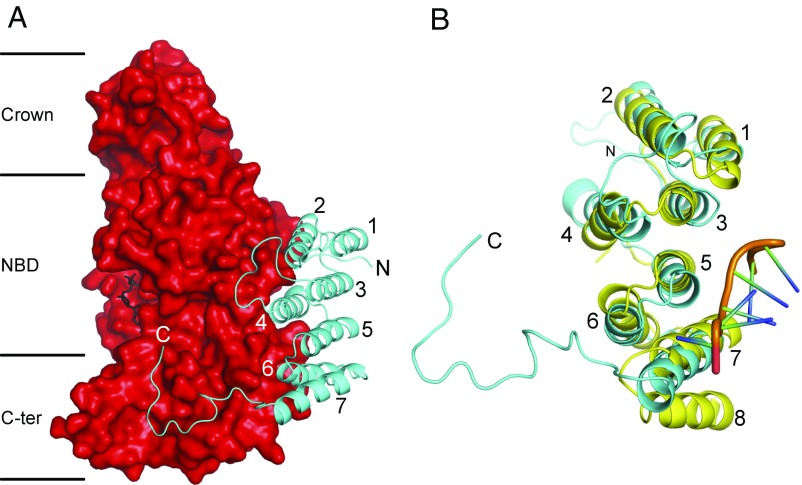
Fig. 3.
Structure of the p18-subunit of the F1-ATPase from T. brucei, and its relation to a PPR protein. (A) A p18 subunit (cyan) in cartoon representation, folded into α-helices H1–H7, with an extended C-terminal region from residues 151–170, bound to the αDP-subunit in solid representation (red). The N-terminal, nucleotide-binding, and C-terminal domains of the α-subunit are indicated by Crown, NBD, and C-ter, respectively; the bound ADP molecule is in black. (B) Comparison of the p18-subunit with an example PPR protein, the PPR10 protein from Z. mays (yellow) (57). PPR10 has 18 PPRs; the structures of PPRs 11–14 are shown (Fig. S3). The orange region represents the backbone of an eight-residue ribonucleotide bound to PPR10. The three PPRs in the p18-subunit correspond to H1 plus H2 (residues 20–28 and 33–45), H3 plus H4 (residues 52–64 and 78–93), and H5 plus H6 (residues 99–112 and 115–126). PPR10 has an additional α-helix, labeled 8, which together with α-helix 7 constitutes a fourth PPR.