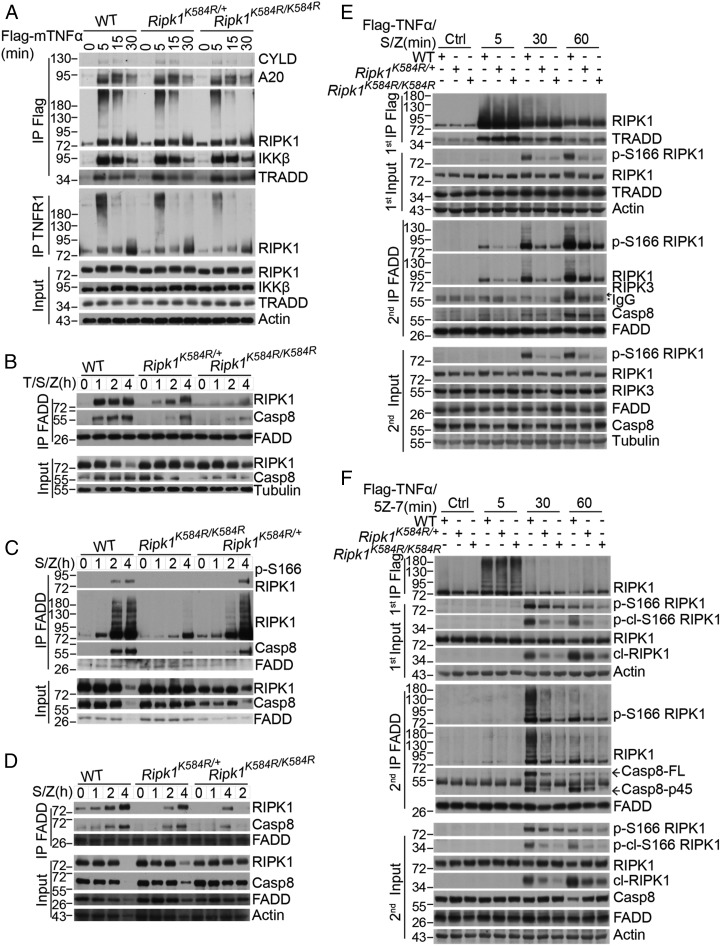
Fig. 3.
The Ripk1K584R mutation disrupts the formation of complex IIa. (A) WT, Ripk1K584R/+, and Ripk1K584R/K584R primary MEFs were treated for 100 ng/mL Flag-TNFα for indicated time points. The cells were lysed with 0.5% Nonidet P-40 buffer and divided equally into two parts for immunoprecipitation with anti-Flag and anti-TNFR1 antibody, respectively. All immunoprecipitated complexes and whole-cell lysates were analyzed by Western blotting with the indicated antibodies. (B–D) WT, Ripk1K584R/+, and Ripk1K584R/K584R primary MEFs (B), primary BMDMs (C), or primary peritoneal macrophages (D) were pretreated with 50 nM SM-164 for 2 h, and then TNFα and zVAD were added as indicated for 1, 2, and 4 h. The cells were lysed with 0.5% Nonidet P-40 buffer and immunoprecipitated with anti-FADD antibody. The immunoprecipitated complex was then analyzed by Western blotting with caspase-8, TRADD, FADD, and RIPK1 antibodies. (E and F) WT, Ripk1K584R/+, and Ripk1K584R/K584R immortalized MEFs were pretreated with 100 nM SM-164 (E) or 500 nM 5Z-7 (F) for 2 h and 50 μM zVAD for 30 min, and then 100 ng/mL TNFα was added for 5, 30, and 60 min. The cells were lysed with 0.5% Nonidet P-40 buffer. The cell lysates were collected and sequentially immunoprecipitated with anti-Flag and anti-FADD antibody. First immunoprecipitation (IP): The TNFR1 complex I was immunoprecipitated using anti-Flag antibody. Second IP: the supernatants after the first IP were then immunoprecipitated with anti-FADD antibody. The immunoprecipitated complexes and whole-cell lysates were analyzed by Western blotting with the indicated antibodies.