Figure 2.
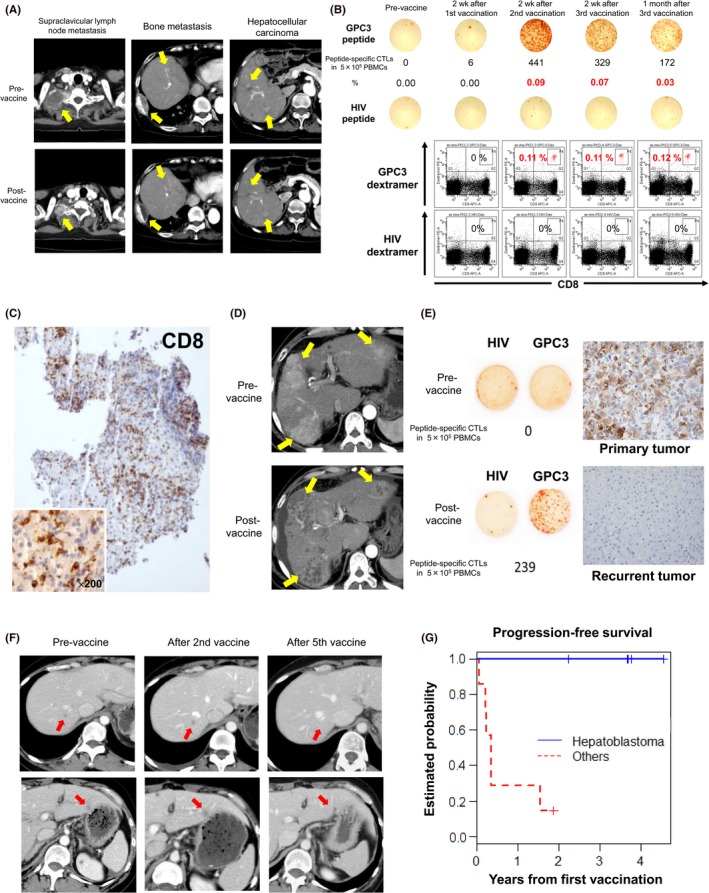
Results from clinical trials of glypican‐3 (GPC3) peptide vaccines. A, Computed tomography scans of a patient with partial response in a phase I trial show remarkable shrinkage of lymph node metastasis and disappearance of two hepatocellular carcinoma (HCC) nodules. Yellow arrows indicate those lesions. B, Peptide‐specific CTLs in peripheral blood, as measured by ex vivo γ‐interferon enzyme‐linked immunospot assay. Top panels indicate that the number of peptide‐specific CTLs in 0.5 million PBMCs increased from 0 to 441 in this patient. Bottom panels show a number of peptide‐specific CTLs after two vaccinations, as measured by flow cytometry of cells stained with GPC3 dextramer. C, Tumor biopsy of an HCC that did not change in size after vaccination, but became infiltrated with a large number of CD8+ killer T cells. D, Computed tomography scan of a case in which almost all HCCs became necrotic after two vaccinations. Yellow arrows indicate those lesions. E, In a clinical trial to prevent HCC recurrence after radical treatment, peptide‐specific CTLs were detected in peripheral blood after vaccination. However, the cancer relapsed thereafter, and was resected. GPC3 expression was observed in the primary tumor before vaccination, but not in the recurrent tumor. F, Computed tomography scans of a patient with ovarian clear cell carcinoma who responded to GPC3 peptide vaccines. After vaccination, all five metastasis sites became inflamed immediately, and disappeared after five vaccinations. Red arrows indicate those lesions. G, Phase I study in pediatric patients with refractory solid tumors. All five patients with hepatoblastoma in remission at enrollment survived and remained in remission by the end of the study
