Abstract
KEYNOTE‐012 was a phase Ib, multicohort study designed to investigate efficacy and safety of pembrolizumab in advanced solid tumors. Results from the subset of patients with recurrent/metastatic head and neck squamous cell carcinoma (HNSCC) from the Asia‐Pacific region are reported. Patients with recurrent/metastatic HNSCC, measurable disease (RECIST version 1.1), and ECOG performance status (PS) 0‐1 were eligible for enrollment in the HNSCC expansion cohort. Patients received pembrolizumab 200 mg every 3 weeks. Response was assessed every 8 weeks. Co‐primary end‐points were safety and overall response rate (RECIST version 1.1, central review). Secondary end‐points included overall survival and response duration. Patients enrolled at any of the five centers throughout the Asia‐Pacific region were included in these analyses. Twenty‐six patients with HNSCC from the Asia‐Pacific region received pembrolizumab. The median age was 62 years, 65% of patients had ECOG PS 1, and 62% had received two or more prior therapies for recurrent/metastatic disease. Sixteen (62%) patients experienced a treatment‐related adverse event of any grade, including two (8%) patients who experienced one or more events of grade 3 severity. No treatment‐related deaths occurred. The overall response rate was 19% (95% confidence interval, 7%‐39%). After a median follow‐up of 12 months (range, 2‐21 months), a median response duration was not reached (range, 6 to 17+ months); four of five responses lasted ≥6 months. Median overall survival was 11.6 months (95% confidence interval, 4.7‐17.7 months). Pembrolizumab was well tolerated and had durable antitumor activity in patients with HNSCC from the Asia‐Pacific region. (Trial registration no. NCT01848834.)
Keywords: Asia‐Pacific, head and neck squamous cell carcinoma, PD‐1, PD‐L1, Pembrolizumab
Abbreviations
- AE
adverse event
- CI
confidence interval
- CPS
combined positive score
- CR
complete response
- DOR
duration of response
- HNSCC
head and neck squamous cell carcinoma
- ORR
overall response rate
- OS
overall survival
- PD
progressive disease
- PD‐1
programmed death‐1
- PD‐L1
programmed death ligand‐1
- PFS
progression‐free survival
- PR
partial response
- PS
performance status
1. INTRODUCTION
Head and neck squamous cell carcinoma poses a significant public health burden in the Asia‐Pacific region. The global incidence of HNSCC tops 600 000 cases annually, with more than half of all cases originating in the Asia‐Pacific region.1, 2, 3 In addition to the traditional HNSCC risk factors (ie, tobacco and alcohol use and human papillomavirus infection),4, 5 other cultural influences, such as the chewing of betel nut, the consumption of products high in nitroso compounds, and exposure to Epstein–Barr virus, contribute to an increase in the occurrence of HNSCC in this region.6, 7, 8
In Asia, a multimodal approach that combines platinum‐based chemotherapy, radiotherapy, and another agent (preferably cetuximab), is recommended for the treatment of patients with recurrent/metastatic HNSCC.9 However, the concomitant use of cisplatin and cetuximab in advanced HNSCC is associated with substantial toxicity,10 and therefore its use is limited in vulnerable populations, such as the elderly.9 Although there are no standard alternatives, other commonly used second‐line treatments (eg, methotrexate or taxanes) are often plagued by low response rates (ranging from 3% to 13%).5, 11 Thus, there is an unmet need in recurrent/metastatic HNSCC for treatment options that are both well tolerated and effective.
The PD‐1 pathway is an important immune checkpoint that has been established as an effective target in HNSCC.12, 13, 14, 15 Pembrolizumab, an anti‐PD‐1 antibody, has shown robust antitumor activity and a manageable safety profile in multiple tumor types, and is currently approved for one or more advanced malignancies, including in the USA for patients with recurrent or metastatic HNSCC with disease progression on or after platinum‐containing chemotherapy.16 This approval was based on efficacy and safety outcomes from the phase Ib multicohort KEYNOTE‐012 trial (ClinicalTrials.gov, NCT01848834).16 KEYNOTE‐012 included two HNSCC cohorts. The initial cohort (n = 60) enrolled only patients with PD‐L1‐positive tumors, and pembrolizumab 10 mg/kg was given every 2 weeks.12 The expansion cohort (n = 132) enrolled patients regardless of PD‐L1 status, and pembrolizumab 200 mg was given every 3 weeks.13 The confirmed ORR in both cohorts was 18%, and responses were durable.12, 13 After a median follow‐up of 14 months and 9 months in the initial and expansion cohorts, respectively, the median DOR was 53 weeks in the initial cohort and was not reached in the expansion cohort.12, 13 Additionally, pembrolizumab was well tolerated in both cohorts, with treatment‐related AEs of grade 3‐4 severity occurring in 17% and 9% of patients, respectively.12, 13
The HNSCC expansion cohort of KEYNOTE‐012 included patients from the Asia‐Pacific region. Here, we report the outcomes of this subgroup of patients.
2. MATERIALS AND METHODS
2.1. Patients
KEYNOTE‐012 was a phase Ib, multicenter, non‐randomized, multicohort trial designed to investigate the safety and efficacy of pembrolizumab in patients with advanced solid tumors.12, 13, 17, 18 The study protocol and all amendments were approved by relevant regulatory and independent ethics committees. The trial was carried out in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written informed consent before entering the study.
Detailed eligibility criteria for the HNSCC expansion cohort have been published elsewhere.13 Briefly, the expansion cohort included adults (age ≥18 years) with histologically or cytologically confirmed recurrent, metastatic, or persistent HNSCC, measurable disease per RECIST version 1.1, ECOG PS of 0 or 1, and adequate organ function. There was no limit to the number of prior therapies, but prior treatment with agents targeting T‐cell co‐stimulation or checkpoint pathways was not allowed. Prior systemic immunosuppressive therapy, small molecule therapy, and anticancer mAb therapy had to be concluded within 7 days, 2 weeks, and 4 weeks before the start of pembrolizumab, respectively. Patients with known additional progressing malignancies, central nervous system metastases, active autoimmune disease, interstitial lung disease, hepatitis B or C, or HIV infection were excluded.
2.2. Study design
Patients in the expansion cohort received pembrolizumab 200 mg i.v. every 3 weeks for 24 months or until confirmed PD, unacceptable toxicity, investigator decision to withdraw the patient, or patient decision to withdraw consent. Tumor imaging was carried out by computed tomography or MRI every 8 weeks, and tumor response was assessed per RECIST version 1.1. Patients with initial radiologic evidence of PD could remain on treatment at the discretion of the investigator until repeat imaging was undertaken no sooner than 4 weeks later. During repeat imaging, if a reduction in tumor burden compared with what was seen on the initial scan was observed, the patient could continue on treatment as scheduled. Patients with confirmed PD discontinued treatment. Patients who achieved an investigator‐determined CR could stop pembrolizumab after receiving at least 24 weeks of treatment. Patients who discontinued after achieving a CR or after the completion of 24 months of pembrolizumab were eligible for up to one additional year of treatment if subsequent progression occurred.
Adverse events were monitored throughout the trial and for 30 days after the end of treatment. Monitoring for serious AEs and immune‐mediated AEs, defined as events with potentially drug‐related immunologic causes regardless of attribution to treatment by the investigator, was continued for 90 days after treatment end. Adverse events were graded according to the NCI's Common Terminology Criteria for Adverse Events version 4.0. Pembrolizumab was withheld for grade 3 treatment‐related AEs and severe AEs until toxicity resolved to grade 0/1. If toxicity did not resolve within 12 weeks, treatment was discontinued. Treatment was also discontinued for grade 4 treatment‐related AEs.
The co‐primary end‐points were safety and ORR per RECIST version 1.1 by central imaging vendor review. Secondary end‐points included ORR by RECIST version 1.1 per investigator assessment, PFS, OS, and DOR.
2.3. Statistical analysis
Efficacy and safety analyses were undertaken in all patients who received one dose or more of pembrolizumab (all‐patients‐as‐treated population). The subgroup analysis presented herein was restricted to patients receiving treatment at a site located in the Asia‐Pacific region.
Exact methods for binomial parameters were used to determine ORR and 95% CIs per RECIST version 1.1. Patients with missing data were considered non‐responders. Kaplan–Meier statistics were used to estimate PFS, OS, and DOR. Patients with missing survival data were censored at their last assessment. Non‐responders were excluded from DOR analyses. The data cut‐off date for these analyses was April 26, 2016.
3. RESULTS
3.1. Patients
In total, 26 patients with HNSCC were enrolled at one of five centers located in the Asia‐Pacific region (National Cancer Center Hospital East, Kashiwa, Japan; Aichi Cancer Center Hospital, Nagoya, Japan; Seoul National University Hospital, Seoul, Korea; Severance Hospital, Seoul, Korea; and National Taiwan University Hospital, Taipei, Taiwan). The median age was 62 years (range, 44‐73 years), 85% of patients were male, and 65% had ECOG PS 1 (Table 1). With regard to traditional HNSCC risk factors, the majority of patients had a history of smoking (73%), whereas only two (8%) patients had human papillomavirus‐associated disease. Patients were heavily pretreated; 61% had received two or more prior therapies for recurrent/metastatic disease and 92% had received prior platinum treatment (Table 1). Twenty‐two (85%) patients had tumors that expressed PD‐L1 per a CPS (number of PD‐L1‐positive cells [tumor cells, lymphocytes, and macrophages] divided by the total number of tumor cells × 100 as determined by immunohistochemistry) ≥1 (previously reported as and equivalent to 1%);13 four (15%) patients had tumors that did not express PD‐L1 (CPS <1). At the data cut‐off date (April 26, 2016), 23 patients had discontinued pembrolizumab because of PD (n = 21) or an AE (n = 2); three patients were continuing on treatment.
Table 1.
Baseline demographics and disease characteristics of patients with advanced head and neck squamous cell carcinoma treated with pembrolizumab (Asia‐Pacific all‐patients‐as‐treated population)
| Characteristic | n = 26 |
|---|---|
| Age, years, median (range) | 62 (44‐73) |
| Male | 22 (85) |
| ECOG PS | |
| 0 | 9 (35) |
| 1 | 17 (65) |
| Smoking history | |
| Current or former | 19 (73) |
| Never | 7 (27) |
| HPV status | |
| HPV‐associated | 2 (8) |
| Non‐HPV‐associated | 24 (92) |
| Sum of target lesions at baseline, mm, median (range) | 108 (21‐269) |
| Primary tumor location | |
| Hypopharynx | 9 (35) |
| Oral cavity | 5 (19) |
| Oropharynx | 4 (15) |
| Larynx | 4 (15) |
| Nasopharynx | 2 (8) |
| Nasal cavity | 1 (4) |
| External auditory canal | 1 (4) |
| Combined positive score ≥1 | 22 (85) |
| Previous adjuvant and/or neoadjuvant therapy | 12 (46) |
| Prior lines of systemic therapy, median (range) | 3 (1‐5) |
| No. of previous lines of therapy for recurrent or metastatic disease | |
| 0 | 4 (15) |
| 1 | 6 (23) |
| 2 | 4 (15) |
| ≥3 | 12 (46) |
| Progressed after platinum treatment | 24 (92) |
HPV, human papillomavirus; PS, performance status.
Data are given as n (%) unless otherwise stated.
Median pembrolizumab exposure at the data cut‐off was 95 days (range, 1‐617 days). Median follow‐up at that time was 12 months (range, 2‐21 months).
3.2. Safety
Sixteen (62%) patients experienced treatment‐related AEs of any grade, the most common of which were fatigue (n = 5), decreased appetite (n = 5), hypothyroidism (n = 4), and rash (n = 4) (Table 2). Two (8%) patients experienced ≥1 grade 3 treatment‐related AEs: one patient had an infection and another experienced decreased appetite and stomatitis (Table 2). Two patients experienced serious treatment‐related AEs, one of which led to the only discontinuation due to a treatment‐related AE (grade 2 interstitial lung disease). There were no treatment‐related deaths. Immune‐mediated AEs, which are reported regardless of attribution to treatment by the investigator, occurred in 5 (19%) patients: grade 2 hypothyroidism (n = 4), grade 3 drug‐induced liver injury (n = 1), grade 2 interstitial lung disease (n = 1), and grade 2 pneumonitis (n = 1).
Table 2.
Treatment‐related adverse events (AEs) by grade severity in patients with advanced head and neck squamous cell carcinoma treated with pembrolizumab (Asia‐Pacific all‐patients‐as‐treated population, n = 26)
| Treatment‐related AE | Any grade occurring in ≥2 patients |
|---|---|
| Patients with ≥1 event | 16 (62) |
| Fatigue | 5 (19) |
| Decreased appetite | 5 (19) |
| Hypothyroidism | 4 (15) |
| Rash | 4 (15) |
| Pyrexia | 3 (12) |
| Dry skin | 2 (8) |
| Lung infection | 2 (8) |
| Anemia | 2 (8) |
| Decreased lymphocytes | 2 (8) |
| Hypercalcemia | 2 (8) |
| Grade 3a occurring in ≥1 patient | |
|---|---|
| Patients with ≥1 event | 2 (8) |
| Stomatitis | 1 (4) |
| Infection | 1 (4) |
| Decreased appetite | 1 (4) |
There were no treatment‐related AEs of grade 4 or 5 severity. Data are shown as n (%).
3.3. Efficacy
At the data cut‐off, five patients had achieved a confirmed PR, for an ORR of 19% (95% CI, 7%‐39%); no patients achieved a CR (Table 3). Another eight (31%) patients achieved stable disease, and 12 (46%) patients had a best response of PD. An additional patient achieved an unconfirmed PR, for an ORR by investigator assessment of 23% (95% CI, 9%‐44%). When the analysis was restricted to patients who progressed after platinum‐based therapy (n = 24), a confirmed ORR of 17% (95% CI, 5%‐37%) was observed (Table 3). When the analysis was restricted to the 22 patients whose tumors expressed PD‐L1 (CPS ≥1), the ORR was 23% (95% CI, 8%‐45%). Overall, a reduction in target lesion size was observed in 50% of patients, and most reductions were maintained over several imaging assessments (Figure 1A,B).
Table 3.
Antitumor activity of pembrolizumab in patients with advanced head and neck squamous cell carcinoma (Asia‐Pacific all‐patients‐as‐treated population)
| Response evaluation | Responses per RECIST version 1.1 by central imaging vendor review | Responses per RECIST version 1.1 by investigator assessment | ||||
|---|---|---|---|---|---|---|
| All patients, n = 26 | Progressed following prior platinum treatment, n = 24 | All patients, n = 26 | ||||
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| ORR | 5 | 19 (7‐39) | 4 | 17 (5‐37) | 6 | 23 (9‐44) |
| CR | 0 | 0 (0‐0) | 0 | 0 (0‐0) | 0 | 0 (0‐0) |
| PR | 5 | 19 (7‐39) | 4 | 17 (5‐37) | 6 | 23 (9‐44) |
| SD | 8 | 31 (14‐52) | 8 | 33 (16‐55) | 9 | 35 (17‐56) |
| PD | 12 | 46 (27‐67) | 11 | 46 (26‐67) | 10 | 39 (20‐59) |
| NAa | 1 | 4 (<1‐20) | 1 | 4 (<1‐21) | 1 | 4 (<1‐20) |
CI, confidence interval; CR, complete response; NA, not assessed; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Patient had no postbaseline imaging.
Figure 1.
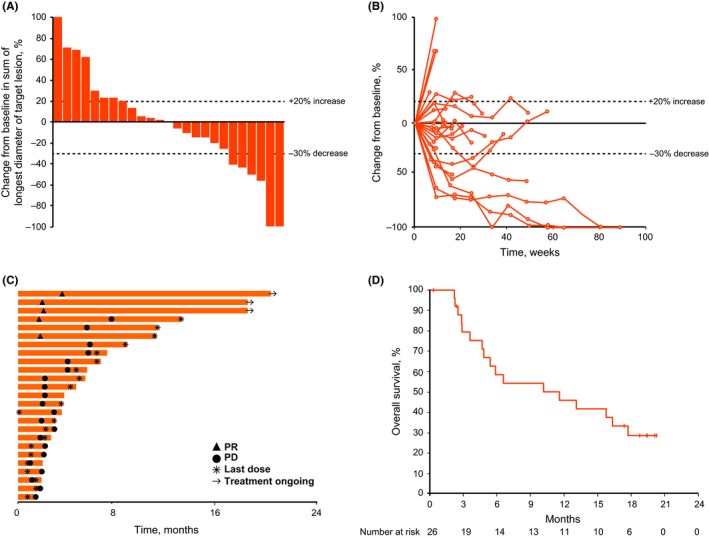
Efficacy of pembrolizumab, based on RECIST version 1.1 by central imaging vendor review. Data shown are the maximum percentage change from baseline in target lesions (n = 25) (A), change from baseline in tumor size over time (n = 25) (B), treatment exposure and response duration (n = 25) (C), and Kaplan–Meier estimate of overall survival (n = 26) (D). PD, progressive disease; PR, partial response
The median time to response was 1.9 months (range, 1.6‐3.5 months) (Figure 1C). At the data cut‐off, the median DOR was not reached (range, 6 to 17+ months), four out of five responses had lasted ≥6 months (Kaplan–Meier estimate), and 80% of responses were ongoing.
The median PFS was 2.1 months (95% CI, 1.9‐5.4). The PFS rates at 6 and 12 months were 20% and 16%, respectively. Median OS was 11.6 months (95% CI, 4.7‐17.7). The 6‐month OS rate was 59%, and the 12‐month OS rate was 46% (Figure 1D).
4. DISCUSSION
Head and neck squamous cell carcinoma is particularly burdensome in the Asia‐Pacific region, where cultural factors combine with traditional risk factors to increase the incidence of HNSCC compared with the rest of the world.1, 4, 5, 6 Pembrolizumab was well tolerated and displayed durable antitumor activity in patients with HNSCC from the Asia‐Pacific region enrolled in the HNSCC expansion cohort of KEYNOTE‐012. The large majority of treatment‐related AEs were of grade 1 or 2 severity. Only two patients experienced a grade 3 treatment‐related AE, and no deaths were attributed to a treatment‐related event. Additionally, the safety profile of pembrolizumab in the Asia‐Pacific subgroup was similar to that reported previously with pembrolizumab in other malignancies, and no new safety risks were identified.17, 18, 19, 20, 21, 22
Efficacy outcomes in the Asia‐Pacific subpopulation were similar to those observed in the total expansion cohort and the initial HNSCC cohort; the ORR among Asia‐Pacific patients was 19%, compared with 18% seen in the expansion and initial cohorts.12, 13 Also similar to both initial and total expansion cohorts, responses were durable in the Asia‐Pacific subpopulation, with the DOR in patients in the Asia‐Pacific region being not reached at the time of data cut‐off, and 80% of responses were still ongoing.12, 13 Therefore, similar to what has been reported previously, the present study results were not impacted by ethnicity, and Asian patients had similar clinical outcomes to the global study population.12, 13, 23
The efficacy and safety of pembrolizumab in patients with HNSCC is similar to what has been reported with another anti‐PD‐1 inhibitor, nivolumab. During the phase III CheckMate‐141 study, 13% of patients with HNSCC responded to nivolumab.10 The frequency and type of AEs seen with nivolumab during CheckMate‐141 were also similar to those observed with pembrolizumab in HNSCC.10 However, the specific outcomes of the Asia‐Pacific subgroup from CheckMate‐141 have not been reported, precluding the comparison of these anti‐PD‐1 inhibitors in that patient population.
Likewise, few studies have reported specifically on treatment outcomes in patients from the Asia‐Pacific region with advanced HNSCC, making it difficult to compare our results with other therapy options. However, the median OS with pembrolizumab in the present study (11.6 months) was similar to that reported in patients from China and Korea who were treated with the recommended first‐line regimen of cisplatin/5‐fluorouracil plus cetuximab (13 months).24 Despite the heavily pretreated population in our study, the toxicity of pembrolizumab was notably better than the platinum and cetuximab regimen (grade 3‐4 treatment‐related AEs, 8% vs 44%).24 A head‐to‐head comparison of pembrolizumab vs this standard first‐line treatment regimen is underway in the phase III KEYNOTE‐048 trial (ClinicalTrials.gov, NCT02358031).
The results presented here add to a growing body of evidence supporting the use of pembrolizumab in patients with HNSCC.12, 13, 15 This subgroup analysis is limited by the small number of patients enrolled from the Asia‐Pacific region, and the results should therefore be interpreted with this limitation in mind. Ongoing phase III trials are being undertaken worldwide, including in the Asia‐Pacific region, to further investigate the efficacy and safety of pembrolizumab in advanced HNSCC. Subgroup analyses of Asia‐Pacific patients in these trials are needed to confirm the clinical utility of pembrolizumab to treat HNSCC in this region of the world.
DISCLOSURE
The study was designed and funded by Merck & Co., Inc. M.T. has received honoraria from Merck Serono. K.M. has received honoraria from Chugai, Takeda, Eli Lilly, Taiho Pharmaceutical, Merck Serono, and YaKult Pharmaceutical and research funding from Merck Sharp & Dohme Corp., Daiichi Sankyo, Ono Pharmaceuticals, Shionogi Pharmaceuticals, Kyowa Hakko Kirin, and Gilead Sciences. K.T. is an employee of MSD K.K. J.D.C. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. and owns stock in the company. Y.J.B. has received research funding from Merck Sharp & Dohme Corp., Bristol‐Myers Squibb, Ono Pharmaceuticals, AstraZeneca, Genentech‐Roche, Merck Serono, Pfizer, and BeiGene. Y.H., H.C.C., C.C.L., and B.K. have no conflict of interest.
ACKNOWLEDGMENTS
Funding for this research was provided by Merck & Co., Inc. (Kenilworth, NJ, USA). The authors thank the patients and their families and caregivers for participating in the study. The authors also thank Kumudu Pathiraja and Roger Dansey, employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., and Yoshinobu Tanaka, Kentaro Imai, Reiko Nagayasu, Ryuichiro Watanabe, Nijiro Nohata, Takashi Shimamoto, and Masaru Watanabe, employees of MSD K.K. Medical writing assistance was provided by Matthew Grzywacz, Dana Francis, and the ApotheCom pembrolizuamb team (Yardley, PA, USA). This assistance was funded by Merck & Co., Inc.
Tahara M, Muro K, Hasegawa Y, et al. Pembrolizumab in Asia‐Pacific patients with advanced head and neck squamous cell carcinoma: Analyses from KEYNOTE‐012. Cancer Sci. 2018;109:771–776. https://doi.org/10.1111/cas.13480
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Gupta B, Johnson NW, Kumar N. Global epidemiology of head and neck cancers: a continuing challenge. Oncology. 2016;91:13‐23. [DOI] [PubMed] [Google Scholar]
- 3. Mahdavifar N, Ghoncheh M, Mohammadian‐Hafshejani A, Khosravi B, Salehiniya H. Epidemiology and inequality in the incidence and mortality of nasopharynx cancer in Asia. Osong Public Health Res Perspect. 2016;7:360‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pelucchi C, Gallus S, Garavello W, Bosetti C, La VC. Cancer risk associated with alcohol and tobacco use: focus on upper aero‐digestive tract and liver. Alcohol Res Health. 2006;29:193‐198. [PMC free article] [PubMed] [Google Scholar]
- 5. Denaro N, Russi EG, Adamo V, Merlano MC. State‐of‐the‐art and emerging treatment options in the management of head and neck cancer: news from 2013. Oncology. 2014;86:212‐229. [DOI] [PubMed] [Google Scholar]
- 6. Liao CT, Chang JT, Wang HM, et al. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann Surg Oncol. 2008;15:915‐922. [DOI] [PubMed] [Google Scholar]
- 7. Fang CY, Huang SY, Wu CC, et al. The synergistic effect of chemical carcinogens enhances Epstein‐Barr virus reactivation and tumor progression of nasopharyngeal carcinoma cells. PLoS ONE. 2012;7:e44810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Relevance of N‐Nitroso compounds to human cancer: Exposers and mechanisms. Proceedings of the IXth International Symposium on N‐Nitroso Compounds; September 1‐5, 1986; Baden, Austria; 1987. [PubMed]
- 9. D'Cruz A, Lin T, Anand AK, et al. Consensus recommendations for management of head and neck cancer in Asian countries: a review of international guidelines. Oral Oncol. 2013;49:872‐877. [DOI] [PubMed] [Google Scholar]
- 10. Vermorken JB, Mesia R, Rivera F, et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116‐1127. [DOI] [PubMed] [Google Scholar]
- 11. Patel AN, Mehnert JM, Kim S. Treatment of recurrent metastatic head and neck cancer: focus on cetuximab. Clin Med Insights Ear Nose Throat. 2012;5:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐012): an open‐label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956‐965. [DOI] [PubMed] [Google Scholar]
- 13. Chow LQ, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker‐unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE‐012 expansion cohort. J Clin Oncol. 2016;34:3838‐3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum‐ and cetuximab‐refractory head and neck cancer: results from a single‐arm, phase II study. J Clin Oncol. 2017;35:1542‐1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Merck & Co., Inc . Keytruda [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2017. [Google Scholar]
- 17. Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE‐012): a non‐randomised, open‐label, phase 1b study. Lancet Oncol. 2017;18:212‐220. [DOI] [PubMed] [Google Scholar]
- 18. Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD‐L1‐positive advanced gastric cancer (KEYNOTE‐012): a multicentre, open‐label, phase 1b trial. Lancet. 2016;17:717‐726. [DOI] [PubMed] [Google Scholar]
- 19. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372:2018‐2028. [DOI] [PubMed] [Google Scholar]
- 20. Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti‐PD‐1) in melanoma. N Engl J Med. 2013;369:134‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robert C, Ribas A, Wolchok JD, et al. Anti‐programmed‐death‐receptor‐1 treatment with pembrolizumab in ipilimumab‐refractory advanced melanoma: a randomised dose‐comparison cohort of a phase 1 trial. Lancet. 2014;384:1109‐1117. [DOI] [PubMed] [Google Scholar]
- 22. Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple‐negative breast cancer: phase Ib KEYNOTE‐012 study. J Clin Oncol. 2016;34:2460‐2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Urba S, Hong RL, Hossain AM, Cheng R, Orlando M. Pemetrexed in combination with cisplatin versus cisplatin monotherapy in East Asian patients with recurrent or metastatic head and neck cancer: results of an exploratory subgroup analysis of a phase III trial. Asia Pacific J Clin Oncol. 2013;9:331‐341. [DOI] [PubMed] [Google Scholar]
- 24. Guo Y, Shi M, Yang A, et al. Platinum‐based chemotherapy plus cetuximab first‐line for Asian patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: Results of an open‐label, single‐arm, multicenter trial. Head Neck. 2015;37:1081‐1087. [DOI] [PubMed] [Google Scholar]


