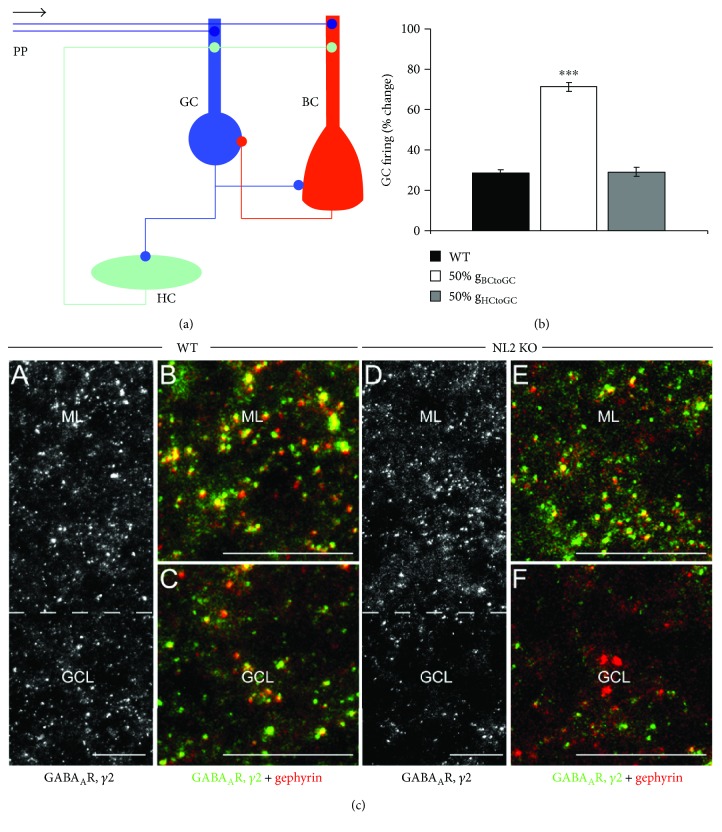
Figure 4.
Biologically detailed network modelling predicted that reduced paired-pulse inhibition of granule cell firing observed in NL2 KO mice was due to the reduction of somatic GABAergic inhibition mediated by basket cell synapses. (a) Schematic circuit depicting connections among excitatory granule cells (GC) and inhibitory basket cells (BC) and hilar cells (HC) in the network model [62]; mossy cells (MC) not shown). (b) Quantification of simulation data on network inhibition of granule cell firing. Network inhibition is weaker and GC firing is significantly higher (t-test, ∗∗∗p < 0.001) in the simulated NL2 KO network model with reduced GABAA conductance (50% reduction of maximum synaptic conductance) at somatic (BC-to-GC) inhibitory synapses (gBCtoGC). Note that no significant impairment of GC network inhibition was observed in the network model with a selective reduction (50%) of GABAA synaptic conductances at dendritic HC-GC synapses (gHCtoGC). (c) The punctate immunostaining of sections from WT (A-C) and NL2 KO mice (D–F) for γ2-subunit of GABAA receptors and inhibitory postsynaptic marker gephyrin. Modelling predicted and experiments confirmed that diminished network inhibition of GC firing, found in electrophysiological recordings in NL2 KO animals, was accompanied by a reduction of somatic GABAA receptor clusters in the granule cell layer (GCL) of the dentate gyrus. Note that the number of GABAA receptor clusters located in the dendritic molecular layer (ML) of the dentate gyrus was not changed in NL2 KO animals. Also, the colocalization of GABAA receptor γ2-subunit and gephyrin was selectively reduced in the GCL but not in the ML of NL2 KO mice (adapted with permission from [59]).