Abstract
Background
Transplantation of human pluripotent stem cell (hPSC)-derived neurons into chick embryos is an established preliminary assay to evaluate engraftment potential. Yet, with recent advances in deriving diverse human neuronal subtypes, optimizing and standardizing such transplantation methodology for specific subtypes at their correlated anatomical sites is still required.
New Method
We determined the optimal stage of hPSC-derived motor neuron (hMN) differentiation for ex ovo transplantation, and developed a single injection protocol that implants hMNs throughout the spinal cord enabling broad regional engraftment possibilities.
Results
A single injection into the neural tube lumen yielded a 100% chick embryo survival and successful transplantation rate with MN engraftment observed from the rostral cervical through caudal lumbar spinal cord. Transplantation of HB9+/ChAT− hMN precursors yielded the greatest amount of engraftment compared to Pax6+/Nkx6.1+/Olig2+ progenitors or mature HB9+/ChAT+ hMNs.
Comparison with existing method(s)
Our single injection hMN transplant method is the first to standardize the optimal hMN phenotype for chick embryo transplantation, provide a rubric for engraftment quantification, and enable broad engraftment throughout the spinal cord with a single surgical intervention.
Conclusion
Transplantation of HB9+/ChAT− hMN precursors into chick embryos of Hamburger Hamilton (HH) stages 15–18 using a single luminal injection confers a high probability of embryo survival and cell engraftment in diverse regions throughout the spinal cord.
Keywords: Regional phenotype, HOX genes, HUES3 HB9::GFP, xenotransplantation, chick embryos
1. Introduction
The number of neuronal subtypes that can be generated from human pluripotent stem cells (hPSCs) has expanded substantially since their first derivation in 2001 (Zhang et al. 2001). Today’s protocols are focused not only on improving the derivation efficiency of novel neuronal subtypes but also refining their phenotypical patterning to distinct anatomical regions of the central nervous system (CNS) (Lemke et al. 2017). Such regional specification has been shown to enhance neuronal engraftment efficiency and regenerative efficacy upon transplantation (Kriks et al. 2011; Ma et al. 2012; Cunningham et al. 2014). However, prior to testing in rodent and large animal studies, transplantation into chick embryos remains a favored preliminary assay to evaluate the hPSC-derived neurons’ engraftment potential (Lee et al. 2007b; Son et al. 2011; Amoroso et al. 2013; Du et al. 2015; Fattahi et al. 2016). Chick embryo transplantation is ideally suited for this type of analysis because it is cost effective, requires minimal regulatory oversight, provides an in vivo microenvironment with developmental cues yet void of a mature immune system, and permits rapid assessment of the transplants’ migratory and engraftment capabilities (Lee et al. 2007a; Wichterle et al. 2009; Boulland et al. 2010; Fattahi et al. 2016). However, customizing and standardizing the methodology to enable optimal engraftment profiles for diverse hPSC-derived neuronal subtypes remains a challenge.
Motor neurons (MNs) reside in discrete columns and pools throughout the hindbrain and spinal cord and project peripherally to provide the sole efferent pathway connecting the CNS to rest of the body. Their diverse regional phenotypes are imparted by colinear and combinatorial expression of 39 HOX genes that regulate the MNs’ columnar and pool identity, positioning within the spinal cord’s ventral horn, and trajectory/connectivity of axonal projections (Philippidou and Dasen 2013). In the first reported derivation from mouse embryonic stem cells (mESCs), in ovo transplantation of spinal MNs was used to demonstrate their functional phenotype (Wichterle et al. 2002). Post-mitotic cervical MNs were derived using 5 days of embryoid body (EB) culture with Retinoic Acid (RA) and Sonic Hedgehog (SHH) morphogenetic patterning for the last 3 days. Then, MN-containing EBs were transplanted into void spaces within the developing posterior neural tube of Hamburger Hamilton (HH) stage 15–18 chick embryos. The void spaces were created by mechanical lesions that physically removed segments of somite and spinal tissues. Over 2–7 days, the transplanted cells engrafted into that focal region and projected axons out of the ventral roots to connect with skeletal muscles. This powerful demonstration of xenotransplantation and engraftment codified the protocol’s methodology (Wichterle et al. 2009), and it has subsequently been used by numerous labs for phenotypical validation of mESC-derived MNs (Soundararajan et al. 2006; Peljto et al. 2010; Toma et al. 2015).
The transition from in ovo transplantation of mESC to hPSC-derived MNs (hMNs) has been accompanied by increased variability in methodological details, partly as a result of inherent differences in derivation protocols. For example, MN derivation from hPSCs versus mESCs is a protracted process, e.g. 2–4 weeks versus 5 days respectively. Hence, the stage of differentiation at which hMNs have been transplanted into the chick embryo’s spinal cord ranges from pluripotency (Goldstein et al. 2002) to HB9+ post-mitotic precursors (Amoroso et al. 2013) to mature, ChAT+ cells (Lee et al. 2007b; Du et al. 2015). Also, both EB/neurosphere (Amoroso et al. 2013; Du et al. 2015) and monolayer (Lee et al. 2007b; Lippmann et al. 2014) hMN derivation protocols are widely used. Thus, hMNs have been transplanted as both spheroids (Amoroso et al. 2013; Du et al. 2015) and cell suspensions (Lee et al. 2007b). Additionally, while transplantation has consistently been performed in HH stage 15–18 embryos, the MNs’ regional phenotype as defined by HOX gene expression, the rostrocaudal transplant location, and the use of a somitic versus midline neural tube lesion has varied amongst different studies (Lee et al. 2007b; Son et al. 2011; Amoroso et al. 2013; Du et al. 2015). Variations in any of these methodological details could significantly affect the efficiency of hMN transplant engraftment. However, evaluating the effects of such protocol alterations is not possible because engraftment results are typically presented as proof-of-principle images instead of being quantitatively assessed. Moreover, these studies demonstrate successful transplantation within only a few focal rostrocaudal spinal regions, while MNs reside at every vertebral segment and derivation of these diverse regional phenotypes from hPSCs is now possible (Gouti et al. 2014; Lippmann et al. 2015). Therefore, there is a need to develop a standardized chick embryo transplantation method that is quantitatively benchmarked and capable of efficiently testing hMN engraftment throughout the entire spinal cord.
To address this need, we have developed a single-injection ex ovo transplantation methodology for hMNs that yielded 100% embryo survival and engraftment at diverse regions throughout the spinal cord. The ex ovo culture system was used to facilitate embryo access and visualization (Yalcin et al. 2010). Also, a rubric was established for quantitative assessment of engraftment levels. HB9+/ChAT− post-mitotic precursors were identified as the optimal differentiation stage for hMN transplantation. Using our deterministic HOX patterning protocol, we specifically transplanted HOXC6 expressing cervical cell populations, and interestingly, observed the greatest levels of engraftment in the embryo’s cervical spinal cord proximal to the wing buds. Collectively, these results provide a standardized and quantitatively benchmarked methodology for effective transplantation of hMNs to achieve broad engraftment throughout the chick embryo spinal cord, thereby obviating the need for multiple focal engraftments as with prior methods. Also, it facilitates hMN engraftment studies investigating whether region-specific (i.e. defined HOX profile) hMN populations display optimal engraftment behaviors at correlated rostrocaudal spinal positions, as suggested by our results.
2. Materials and Methods
2.1 Animal Subjects
Fertilized chick embryos were obtained from Sunnyside Hatchery (Beaver Dam, WI). Upon receipt, the chick embryos were placed with their apex vertical and placed inside a humidified egg incubator set to 37°C. The eggs were allowed to develop for up to 72 hours or until HH stage 15–18.
2.2 hPSC Culture and Motor Neuron Differentiation
The HUES3 HB9::GFP cell line (Harvard Stem Cell Institute) was used for all experiments and maintained in E8/feeder free conditions as described elsewhere (Lippmann et al. 2014). The cells were cultured on Matrigel™ coated plates and routinely passaged with Versene (ThermoFisher). Motor neuron (MN) differentiation was also conducted as previously described (Fig. 1A) (Lippmann et al. 2015). In brief, hPSCs were seeded onto Matrigel-coated 6-well plates at a density of 1.5×105 cells/cm2 in E8 media (ThermoFisher) with 10μM Y-27632 (ThermoFisher). The culture media was changed to E6 media (ThermoFisher) on Day 0, and then changed to E6 media containing 200ng/ml FGF8b (PeproTech) the following day. On Day 2, the cells were gently subcultured and re-seeded at a 2:3 ratio in E6 media containing 10μM Y-27632, 200ng/ml FGF8b and 3μM CHIR99021 (CHIR, R&D Systems). On Day 4, the media was changed to E6 media containing 1μM retinoic acid (RA, Sigma Aldrich), 2μM Purmorphamine (PM, R&D Systems), and 2μM SAG (R&D Systems) for 48 hours to pattern a cervical, Pax6+/Nkx6.1+/Olig2+ MN progenitor phenotype by Day 6. For continued differentiation, the cells were subcultured and re-seeded at a 1:12 ratio in a 12-well plate with E6 media containing 1μM RA and 10μM Y-27632. On Day 7–9, the media was changed daily and supplemented with 100nM PM and 100nM SAG. On Days 10–15, the media was further supplemented with 5μM DAPT (R&D Systems) to generate post-mitotic, HB9+/ChAT− MN precursors by Day 16. For maturation, the cultures were maintained for two additional weeks in E6 media supplemented with neurotrophic factors (NTFs) including 10ng/mL BDNF (PeproTech), 10ng/mL GDNF (PeproTech), N-2 and B-27 supplement (ThermoFisher), and cAMP (Sigma) as well as Glutamax and PenStrep (ThermoFisher). The media was changed every third day and yielded HB9+/ChAT+ MNs by Day 30. Each culture’s regional patterning along the spinal cord’s rostrocaudal axis was evaluated by RT-PCR using HOXA1, HOXB4, HOXC6, HOXC9, and HOXD10 Taqman gene expression assays (Table S1).
Fig. 1.
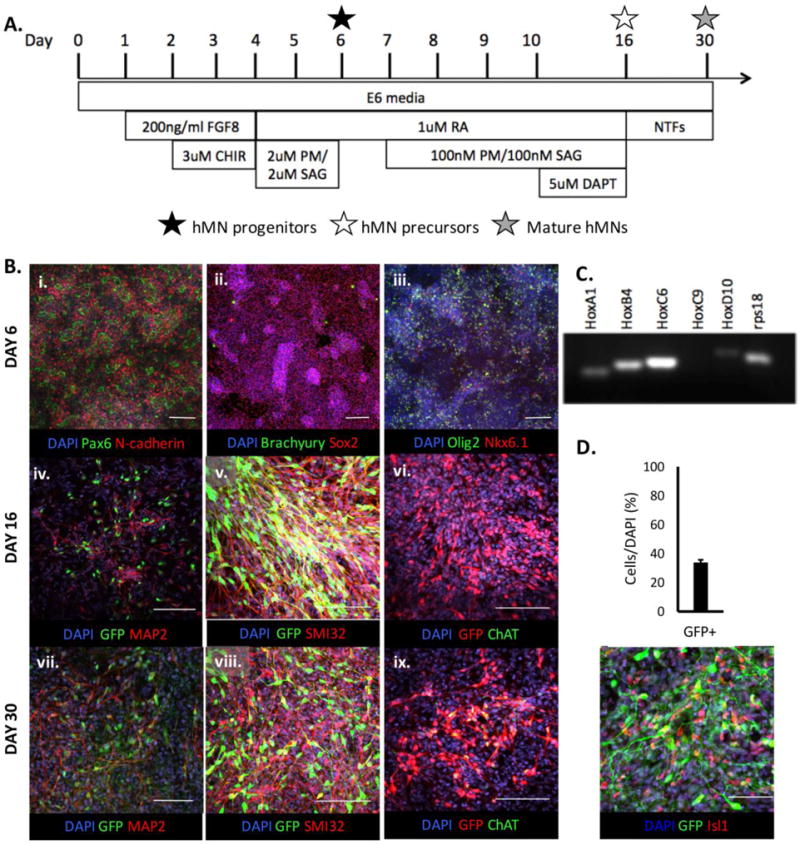
Derivation of hMN cultures. (A) Timeline of derivation protocol. (B) Immunostaining of hMN progenitor (Day 6), precursor (Day 16), and mature neuronal cultures (Day 30). Scale bars are 150 (i, ii, iii, iv) and 100μm (v, vi, vii, viii, ix). (C) RT-PCR analysis of the cultures’ HOX expression profiles on Day 21. Faint bands for HOXA1 and HOXD10 are off target amplifications and not true gene expression (see Table S1). (D) Quantification of GFP+ hMNs and co-staining with ISL1 (n=5 technical replicates). Scale bar is 100μm.
2.3 Microinjection set-up
All microinjection equipment was purchased from Sutter Instruments. For each injection, a 500μL syringe was inserted into the Xenoworks® Analog Microinjector (Cat No. V001182) holder and connected to a custom glass micropipette (PIP50BV30) through tubing and an adapter. Then, the entire assembly (micropipette, tubing, and syringe) was filled with sterile mineral oil to facilitate injection pressure control. Next, the micropipette was mounted with an adapter to a motorized micromanipulator (Cat No. MP-225), and the manipulator was positioned to view the micropipette tip underneath a Leica DMS1000 Digital Stereo Microscope (W. Nuhsbaum Inc.).
2.4 Ex Ovo Chick Embryo Preparation
On the day of transplantation, the eggs were turned horizontal to facilitate transfer by repositioning the yolk. Ex ovo culture of the chick embryo was conducted as previously described in detail (Yalcin et al. 2010). In brief, an embryo culture ‘hammock’ was created using a 9oz plastic cup covered with a piece of GLAD® cling wrap, which drooped a quarter of the way down the cup, secured by a rubber band. The hammocks were sterilized by wiping with 70% ethanol. Next, the eggs were cracked into the hammocks, and Pelican Fount India Ink solution, made by dissolving 10 drops of India Ink in 50mL of sterile Ringers solution (VWR) with 1X Penstrep (Life Technologies), was injected under the embryo with a small hypodermic needle. This increased contrast for embryo visualization under the stereo miscroscope. Then, a flame-polished tungsten needle (Roboz) was used to tear away the vitelline membrane on top of somites 18–19 uncovering the developing neural tube (Fig. 2B). At this same site, the tungsten needle was also used to make a midline lesion in the neural tube slightly enlarging the central canal to facilitate cell transplantation. Afterwards, the embryos were placed back into the incubator and covered with a petri dish lid.
Fig. 2.
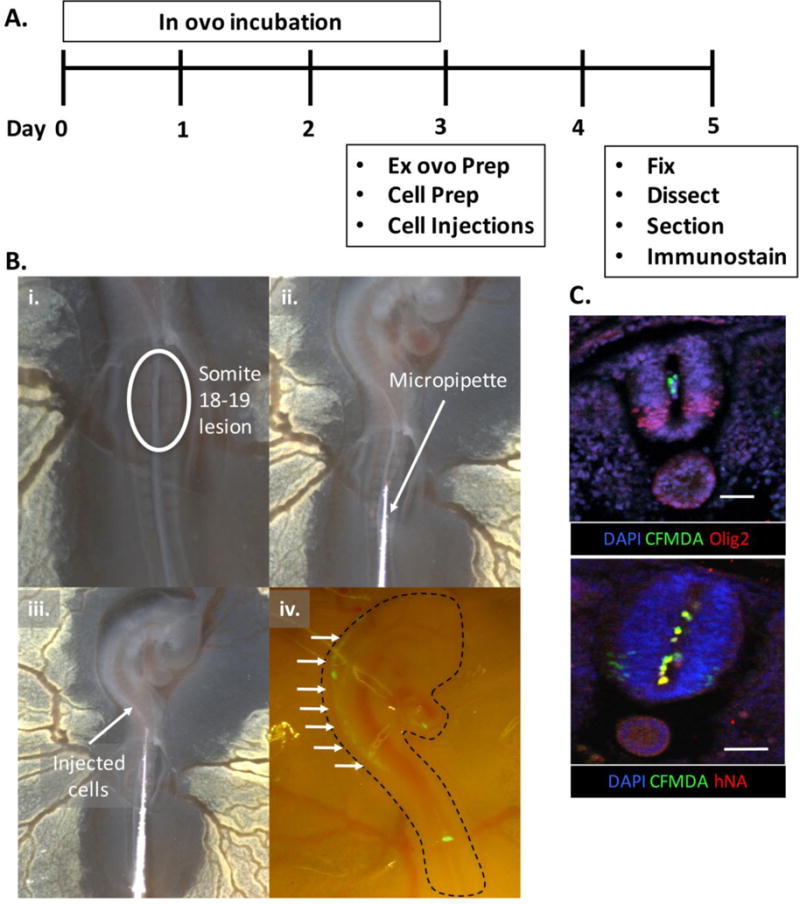
Ex ovo transplantation method. (A) Timeline of experimental protocol with (B) images depicting the (i.) midline neural tube lesion, (ii. and iii.) injection of hMN cultures, and (iv.) visualization of CFMDA dyed cells (arrows) under a stereo microscope immediately afterwards. (C) Immunostained Olig2+ spinal tissue sections (top) showing transplanted cells positive for CFMDA dye and human nuclear antigen (hNA, bottom) in the central canal. Scale bars are 50μm.
2.5 Cell Transplantation
Human MN cultures were labeled with CellTracker™ CFMDA or CMPTX dye (ThermoFisher) prior to transplantation. A 10mM dye stock solution in DMSO (Sigma) was prepared and stored at −20°C protected from light. For cell labeling, the stock solution was diluted to 1mM in PBS and added to the culture medium to achieve a 10μM concentration for 1 hour. This was followed by PBS washes and subculture using a 3-minute incubation in Accutase (Sigma) at 37°C. Then, the cells were centrifuged at 1000rpm for 5 min and resuspended in 100μL of sterile Hank’s Balanced Salt Solution (HBSS, ThermoFisher). This was transferred to a microcentrifuge tube and 5μL of the cell suspension was aspirated into the glass micropipette. The cells were allowed to settle and concentrate at the micropipette outlet. Then, it was inserted at a 30° angle from the horizontal plane into the lesion site at somite 18–19 from the posterior end of the embryo using the micromanipulator. The micropipette tip was advanced rostrally ~2 somites to ensure insertion and create a seal. Next, the cells were injected by gently turning the knob on the microinjector, and they flowed into the neural tube lumen to span the entire posterior neuraxis of the embryo rostral to the injection site. The embryos were imaged immediately using a Leica DMS1000 Digital (brightfield) or Zeiss Dicovery.V8 (fluorescence; Carl Zeiss Microscopy) Stereo Microscope and placed back in the incubator for two additional days prior to analysis.
2.6 Embryo Dissection and Tissue Sectioning
To aid in separation of the embryo from the yolk, a circular piece of Whatman filter paper with a concentric hole was placed on top of the embryo. The vitelline membrane around the filter paper’s exterior was cut away using microdissection scissors, and the embryo was excised by lifting the filter paper with tweezers. This was transferred to a 4% paraformaldehyde solution and fixed overnight at 4°C. Then, the embryo was transferred to PBS with 15% sucrose, and after sinking in the cryoprotectant over the course of a 1–2 days, it was embedded in optical cutting temperature (O.C.T) compound (ThermoFisher) and frozen at −80°C. Next, the entire embryo was sectioned into 40μm thick transverse slices using a Leica CM3050 cryostat. The slices were placed onto Superfrost™ Plus Microscope slides (ThermoFisher) and stored at −20°C for immunostaining.
2.7 Immunostaining
Immunostaining of MN cultures was completed as detailed elsewhere (Lippmann et al. 2015) using primary antibodies (Table S2). For tissue section staining, the slides were allowed to equilibrate to room temperature before being placed back-to-back in coupling jars. Then, they were rinsed 5 × 10 minutes in Tris-buffered saline (TBS) to remove the O.C.T. compound. Afterwards, slides were removed from the coupling jar, and the immobilized tissues on each slide were encircled using an ImmEdge™Hydrophobic Barrier pen (Vector Laboratories). Primary blocking was conducted by pipetting TBS-DT (1× TBS, 0.3% Triton-X, and 5% Donkey serum) onto each slide covering all tissues within the hydrophobic barrier and incubating for 1 hour at room temperature. Next, primary antibody, diluted in TBS-DT at concentrations indicated in Table S2, was added to each slide and incubated at 4°C for 3 days. During this period, the slides were checked occasionally to ensure the tissues were moist at all times and excess TBS-DT was added as needed. After primary staining, the slides were washed 5 × 15 minutes with TBS-T followed by a two-hour incubation in Alexa Flour secondary antibodies (Life Technologies) diluted (1:500) in TBS-DT at room temperature. Then, the slides were rinsed 2 × 10 minutes with TBS, 1 × 10 minutes with a 2.5μg/mL 4′,6-Diamidino-2-Phenylindole (DAPI, ThermoFisher) solution in TBS, and 2 × 5 minutes with TBS. The slides were allowed to dry completely while ensuring that the tissues stayed moist, and mounting was initiated by applying ProLong™ Gold Antifade reagent (ThermoFisher) to the slides and covering with a No. 1 glass coverslide. The slides were dried overnight at room temperature, and nail polish was added to the coverslide/microscope slide edges to seal in the mounted tissues. Slides were stored at −20°C and imaged using a Nikon A1R confocal microscope.
3. Results
3.1 Derivation of hPSC-derived spinal motor neurons
In previous publications, the regional phenotype and differentiation stage of hMNs transplanted into chick embryos has been highly variable (Goldstein et al. 2002; Lee et al. 2007b; Amoroso et al. 2013; Du et al. 2015). In order to standardize and optimize a methodology, we focused on evaluating the engraftment potential of just cervical cells at three different stages of hMN differentiation. Cultures were derived from the HUES3 HB9::GFP line, which is engineered with a GFP transgene under control of a murine Hb9 promoter that fluorescently labels MNs of both medial and lateral motor column phenotypes when differentiated in vitro (Di Giorgio et al. 2008; Amoroso et al. 2013). Using our deterministic HOX patterning protocol (Lippmann et al. 2015), HOXC6 expressing cultures of cervical spinal MN progenitors (Pax6+/Nkx6.1+/Olig2+, Day 6), post-mitotic precursors (GFP+/ChAT−, Day 16), and mature neurons (GFP+/ChAT+, Day 30) were derived (Fig. 1A–C). Analysis of Day 21 cultures revealed a 32.5±1.7% differentiation efficiency of GFP+/Isl1+ and GFP+/Isl1− MNs (Fig. 1D), which is consistent with prior studies using the HUES3 HB9::GFP line (Amoroso et al. 2013). Given this validation of substantial MN differentiation, the progenitor, post-mitotic precursor, and mature neuronal cultures were further evaluated in transplantation studies.
3.2 Ex Ovo Transplantation of hMNs
A consistent practice across prior chick embryo transplantation methods is the injection of hMNs into chick embryos at HH stages 15–18 (Lee et al. 2007b; Son et al. 2011; Amoroso et al. 2013; Du et al. 2015). At these stages, the developing spinal cord is identifiable and readily accessible, flanked by 24–36 somites, and both wing and leg buds have initiated formation (Hamburger and Hamilton 1951). Using this precedent, we investigated whether injecting cells through a midline neural tube lesion would transplant cells throughout the developing spinal cord’s central canal (Fig. 2A). This could potentially facilitate engraftment of hMNs along the spinal cord’s entire rostrocaudal axis instead of only at focal sites as in prior studies (Lee et al. 2007b; Amoroso et al. 2013; Du et al. 2015). The midline lesion was made at somites 18–19, which correlates to the cervical, wing bud region of the spinal cord (Chevallier et al. 1977) (Fig. 2B). Day 6, hMN progenitor cultures were pre-labeled with green CFMDA dye, and microinjected as a cell suspension through the lesion. The cells filled rostrally into the spinal canal reaching the approximate hindbrain-midbrain boundary as observed with a fluorescent stereo microscope. To further verify that the cells resided within the posterior neural tube’s central lumen, the embryos were immediately fixed, cryosectioned along the transverse plane, and spinal sections were analyzed by immunostaining (Fig. 2C). Confocal images showed that spinal tissues, which display a ventral band of endogenous Olig2+ MN progentiors, contained CFMDA-labeled cells that co-stained with human nuclear antigen (hNA+) within their central lumen. This demonstrates that a single microinjection through a midline neural tube lesion is capable of initially distributing implanted cells throughout diverse regions of the developing hindbrain and spinal cord’s central canal rostral to the injection site.
To our knowledge, there is no precedent demonstrating that a cell suspension of hMNs can effectively engraft when transplanted into the spinal cord’s central canal. The closest methodological equivalent transplanted single EBs containing mESC-derived MNs into the spinal cord’s lumen (Soundararajan et al. 2006; Son et al. 2011). Hence, transplantation of hMN progenitor (Day 6), post-mitotic precursor (Day 16), and mature neuronal (Day 30) cell suspensions were tested to increase our probabilities of success (Fig. 1). Each culture was pre-labeled with red-CMPTX dye, injected into 10 different chick embryos, and allowed to engraft over the course of 48 hours. All embryos survived the post-injection incubation period, and subsequent analysis of cryosectioned tissues revealed that 100% of the embryos contained CMPTX+ cells. This included a significant number of sections containing CMPTX+/GFP+ cells in the spinal tissue parenchyma projecting GFP+ axons, thereby demonstrating our cell suspensions transplantation methodology can yield successful hMN engraftment (Fig. 3A). However, there were apparent differences between the level of engraftment observed based on the transplanted culture’s MN differentiation stage.
Fig. 3.
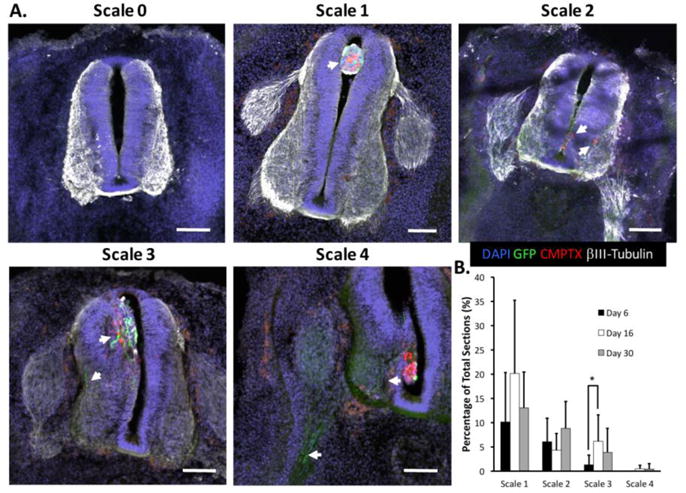
Rubric for hMN engraftment quantification. (A) Scale 0–4 levels of CMPTX+/GFP+ hMN engraftment 48 hrs post-transplantation: no cells present (Scale 0); cells only in central canal (Scale 1); cells migrated into neural tube parenchyma (Scale 2, arrows); cells project GFP+ axons (Scale 3, arrows); cells project GFP+ axons into the ventral root (Scale 4, arrows). Scale bars are 100μm. (B) Quantification of average percentage of tissue sections with Scale 1–4 engraftment (n=10 embryos per experimental group). * indicates p≤0.05 using a Wilcoxon Rank Sum Test with a post-hoc Benjamini-Hochberg procedure.
3.3 Quantification of hMN Engraftment
Since it is impossible to normalize the number of successfully transplanted cell in each embryo, a 5-level scale was created to quantitatively describe the engraftment observed in each tissue section across all embryos (Fig. 3A). This allows normalization of engraftment statistics across experimental groups as well as correlation of engraftment with specific rostrocaudal spinal regions. The lack of any CMPTX+/GFP+ cells or observation of their presence only within the central canal was graded as ‘Scale 0’ or ‘Scale 1’ engraftment, respectively. A ‘Scale 2’ engraftment was assigned when transplanted cells were observed in the spinal tissue parenchyma but no GFP+ projections were detected. If transplanted cells migrated into the spinal tissue parenchyma and projected GFP+ axons either proximally or distally through the ventral roots, then they were respectively graded as ‘Scale 3’ or ‘Scale 4’ engraftments. In our rubric, both Scale 3 and 4 represent successful hMN engraftment but Scale 4 is the ideal scenario.
Quantification revealed significant differences in engraftment based on the transplanted culture’s stage of MN differentiation. Embryos transplanted with HB9+/ChAT− hMN precursors (Day 16) contained the highest percentage of tissue sections on average that displayed successful hMN engraftment (Fig. 3B). This was significantly higher than embryos transplanted with Pax6+/Nkx6.1+/Olig2+ hMN progenitors (Day 6), and embryos transplanted with HB9+/ChAT+ mature hMNs (Day 30) trended towards higher percentages of tissue sections displaying unsuccessful Scale 2 engraftment. Moreover, while the HOXC6 expressing cultures collectively engrafted broadly throughout the spinal cord’s rostrocaudal axis, only post-mitotic hMN precursors demonstrated a significant preference for cervical engraftment proximal to the wing bud (Fig. 4). Thus, microinjecting a cell suspension of HB9+/ChAT−, post-mitotic hMN precursors through a midline neural tube lesion produced optimal engraftment that was spatially correlated with its HOX-dependent regional phenotype.
Fig. 4.
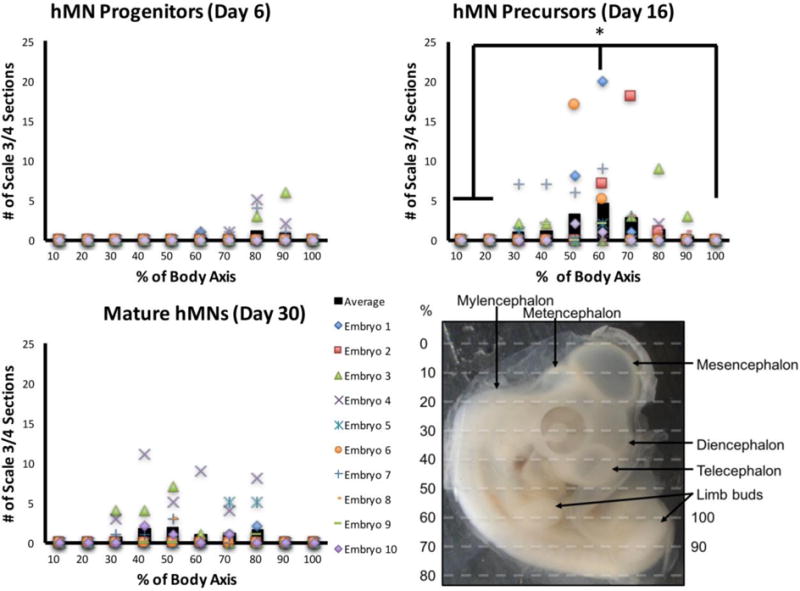
Quantitative analysis of hMN engraftment. Rostrocaudal body axis of chick embryo was subdivided into 10 domains (bottom right image). Each graph shows the number of tissue sections per domain per embryo that displayed Scale 3 or 4 engraftment of GFP+ hMNs. Black bars indicate the average across all 10 embryos, and symbols represent data points for each embryo. Only HB9+(GFP+)/ChAT− hMN precursors showed enhanced engraftment at ~60% of rostrocaudal axis corresponding to the cervical wing bud (n=10 embryos). * indicates p≤0.05 using a Wilcoxon Rank Sum Test with a post-hoc Benjamini-Hochberg procedure.
4. Discussion
The single-injection, ex ovo transplantation methodology described here implants hMNs throughout the spinal cord’s entire rostrocaudal axis. Broad Scale 3/4 engraftment was observed in diverse spinal cord regions, and transplantation of HB9+/ChAT− hMN precursors yielded the highest level of successful engraftment compared to Pax6+/Nkx6.1+/Olig2+ progenitors and HB9+/ChAT+ mature neurons (Fig. 3B and 4). Also, the transplantation procedure was well tolerated as evidenced by a 100% rate of embryo survival 48 hours post-transplant. Thus, we have developed an efficient, standardized, and quantitative methodology for evaluating the engraftment potential of hMN cultures by defining a pan-spinal transplantation technique, the optimal phenotype for transplantation, and a rubric for engraftment quantitation. This will facilitate investigating whether regional specification of hMNs, as determined by their HOX profiles (Lippmann et al. 2015), affects their preferred rostrocaudal region of engraftment and trajectory of axonal projections.
Our results suggest that cervical, HOXC6 expressing hMN precursors preferentially engraft in the chick embryo’s cervical spinal cord (Fig. 4). However, these results are not conclusive and our methodology could still be improved to further facilitate such investigations for hMNs and potentially other spinal cell phenotypes. The observed increase in engraftment of HOXC6 expressing HB9+/ChAT− hMN precursors at ~ 60% of the embryo’s rostrocaudal axis, which corresponded with the wing bud, could have been an artifact of our transplantation methodology. This rostrocaudal position corresponds approximately with the midline lesion location, and similar lesions have been used to mediate engraftment in other hMN transplantation protocols (Son et al. 2011). The neural tube damage inflicted by the lesion could increase local engraftment. Therefore, we cannot rule out that this biased the hMN’s rostrocaudal engraftment location, although a similar phenomenon was not observed when transplanting Pax6+/Nkx6.1+/Olig2+ hMN progenitors or HB9+/ChAT+ mature neurons. Future experiments should test multiple rostrocaudal injection locations for the same cell phenotype to definitively assess any preferential engraftment behaviors.
In our experiments, hMN engraftment 48 hours post-injection was observed throughout the posterior neural tube spanning from ~30–90% of the rostrocaudal axis (Fig. 4). While this pan-spinal engraftment from a single injection is a major advantage of our methodology, we do not fully understand how cells were positioned to engraft caudal to the brachial injection/lesion site. Upon removal of the micropipette after injection, release of backpressure in the spinal canal due to presumed displacement of cerebral spinal fluid (CSF) was observed to re-position a portion of the injected cells caudally towards the lesion site. Also, since the posterior CNS is only partially formed at HH stage 15–18, it could be postulated that cells were further displaced caudally as the thoracic, lumber, and sacral spinal canals fully form and became filled with CSF. While the distribution of cells displaced caudally by such mechanisms pre-engraftment is not known, Scale 3/4 graded sections were observed at 60–90% rostrocaudal axial positions in 14 out of 30 injected embryos.
Although Scale 3/4 engraftment was observed in 73.3% of all transplanted embryos, the absolute number of tissue sections receiving this grade was only ~5% for HB9+/ChAT− hMN precursors (Fig. 3B). These numbers could be depressed due to the fact that only GFP+ cells contributed to this quantification and they accounted for only 32.5±1.7% of transplanted cells (Fig. 1D). However, this could also be indicative of a low level of hMN survival throughout our transplantation procedure. When EBs or neurospheres containing hMNs engraft into the chick spinal cord, robust GFP+ axonal projections from hundreds of neurons are observed within the focal transplant region (Son et al. 2011; Amoroso et al. 2013; Du et al. 2015). In contrast, our injections of hMN suspensions resulted in tissue sections that contained only a few GFP+ axonal projections (Fig. 3A). While this permits better visualization of axonal trajectories within a single tissue section, it also increases the number of embryos required to statistically evaluate projections from enough GFP+ cells. Thus, future improvements to our single-injection, ex ovo transplantation methodology should focus on increasing transplanted cell survival and thereby the absolute number of engrafted hMNs.
Supplementary Material
Highlights.
Single injection transplants cells throughout the chick embryo’s spinal cord lumen.
hPSC-derived, HB9+/ChAT−, motor neuron precursors are optimal for chick transplant.
Transplanted motor neurons display broad engraftment throughout the spinal cord.
Acknowledgments
We thank Prof. James Thompson and Dr. Katie Vermillion for use of their cyrostat and Discovery.V8 stereo microscope and assistance with chick embryo culture technique, respectively. We would like to acknowledge Prof. Sushmita Roy for guidance in conducting nonparametric statistical analyses on engraftment data. Also, we thank Joshua Plantz for experimental assistance on several occasions.
Funding
This publication was developed, in part, using the Assistant Agreement No. 83573701 awarded by the U.S. Environmental Protection Agency (EPA) to R.S.A. and has not been formally reviewed by the EPA. The views expressed in this document are solely those of R.S.A. and colleagues and do not necessarily reflect those of the Agency, and the EPA does not endorse any products or commercial services mentioned in this publication. The publication was also developed with support from NIH grant R21NS082618 and R33NS082618, an Innovation in Regulatory Science Award from the Burroughs Wellcome Fund (R.S.A.), and a UW-Madison Hilldale Fellowship (A.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
References
- Amoroso MW, Croft GF, Williams DJ, O’Keeffe S, Carrasco MA, Davis AR, et al. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J Neurosci. 2013;33(2):574–86. doi: 10.1523/JNEUROSCI.0906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulland J-L, Halasi G, Kasumacic N, Glover JC. Xenotransplantation of human stem cells into the chicken embryo. JoVE. 2010;(41) doi: 10.3791/2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier A, Kieny M, Mauger A. Limb-somite relationship: origin of the limb musculature. Development. 1977 [PubMed] [Google Scholar]
- Cunningham M, Cho J-H, Leung A, Savvidis G, Ahn S, Moon M, et al. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell. 2014;15(5):559–73. doi: 10.1016/j.stem.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human Embryonic Stem Cell-Derived Motor Neurons Are Sensitive to the Toxic Effect of Glial Cells Carrying an ALS-Causing Mutation. Cell Stem Cell. 2008;3(6):637–48. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Du Z-W, Chen H, Liu H, Lu J, Qian K, Huang C-L, et al. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat Commun. 2015;6:6626. doi: 10.1038/ncomms7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattahi F, Steinbeck JA, Kriks S, Tchieu J, Zimmer B, Kishinevsky S, et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531(7592):105–9. doi: 10.1038/nature16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RS, Drukker M, Reubinoff BE, Benvenisty N. Integration and differentiation of human embryonic stem cells transplanted to the chick embryo. Dev Dyn. 2002;225(1):80–6. doi: 10.1002/dvdy.10108. [DOI] [PubMed] [Google Scholar]
- Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, et al. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 2014;12(8):e100. doi: 10.1371/journal.pbio.1001937. 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88(1):49–92. [PubMed] [Google Scholar]
- Kriks S, Shim J-W, Piao J, Ganat YM, Wakeman DR, Xie Z, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–51. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Shamy Al G, Panagiotakos G, Barberi T, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25(12):1468–75. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, et al. Directed Differentiation and Transplantation of Human Embryonic Stem Cell‐Derived Motoneurons. Stem Cells. 2007b;25(8):1931–9. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- Lemke KA, Aghayee A, Ashton RS. Deriving, regenerating, and engineering CNS tissues using human pluripotent stem cells. Curr Opin Biotechnol. 2017;47:36–42. doi: 10.1016/j.copbio.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann ES, Estevez-Silva MC, Ashton RS. Defined human pluripotent stem cell culture enables highly efficient neuroepithelium derivation without small molecule inhibitors. Stem Cells. 2014;32(4):1032–42. doi: 10.1002/stem.1622. [DOI] [PubMed] [Google Scholar]
- Lippmann ES, Williams CE, Ruhl DA, Estevez-Silva MC, Chapman ER, Coon JJ, et al. Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Rep. 2015;4(4):632–44. doi: 10.1016/j.stemcr.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, et al. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10(4):455–64. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peljto M, Dasen JS, Mazzoni EO, Jessell TM, Wichterle H. Functional Diversity of ESC-Derived Motor Neuron Subtypes Revealed through Intraspinal Transplantation. Cell Stem Cell. 2010;7(3):355–66. doi: 10.1016/j.stem.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidou P, Dasen JS. Hox genes: choreographers in neural development, architects of circuit organization. Neuron. 2013;80(1):12–34. doi: 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, et al. Conversion of Mouse and Human Fibroblasts into Functional Spinal Motor Neurons. Cell Stem Cell. 2011;9(3):205–18. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundararajan P, Miles GB, Rubin LL, Brownstone RM, Rafuse VF. Motoneurons derived from embryonic stem cells express transcription factors and develop phenotypes characteristic of medial motor column neurons. J Neurosci. 2006;26(12):3256–68. doi: 10.1523/JNEUROSCI.5537-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma JS, Shettar BC, Chipman PH, Pinto DM, Borowska JP, Ichida JK, et al. Motoneurons derived from induced pluripotent stem cells develop mature phenotypes typical of endogenous spinal motoneurons. J Neurosci. 2015;35(3):1291–306. doi: 10.1523/JNEUROSCI.2126-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110(3):385–97. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Peljto M, Nedelec S. Xenotransplantation of embryonic stem cell-derived motor neurons into the developing chick spinal cord. Methods Mol Biol. 2009;482:171–83. doi: 10.1007/978-1-59745-060-7_11. (Chapter 11) [DOI] [PubMed] [Google Scholar]
- Yalcin HC, Shekhar A, Rane AA, Butcher JT. An ex-ovo chicken embryo culture system suitable for imaging and microsurgery applications. JoVE. 2010;(44) doi: 10.3791/2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129–33. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


