Abstract
The kidneys have a close functional relationship with other organs especially the lungs. This connection makes the kidney and the lungs as the most organs involved in the multi-organ failure syndrome. The combination of acute lung injury (ALI) and renal failure results a great clinical significance of 80% mortality rate. Acute kidney injury (AKI) leads to an increase in circulating cytokines, chemokines, activated innate immune cells and diffuse of these agents to other organs such as the lungs. These factors initiate pathological cascade that ultimately leads to ALI and acute respiratory distress syndrome (ARDS). We comprehensively searched the English medical literature focusing on AKI, ALI, organs cross talk, renal failure, multi organ failure and ARDS using the databases of PubMed, Embase, Scopus and directory of open access journals. In this narrative review, we summarized the pathophysiology and treatment of respiratory distress syndrome following AKI. This review promotes knowledge of the link between kidney and lung with mechanisms, diagnostic biomarkers, and treatment involved ARDS induced by AKI.
Keywords: Acute kidney injury, Acute lung injury, Renal failure, Respiratory distress syndrome, Multiple organ failure
Introduction
The kidneys receive more cardiac output on a per-gram basis than some other organs such as the liver (approximately 25% of cardiac output). Therefore, kidneys are constantly exposed to small peptides and immune regulatory molecules, which can reabsorb these substances from circulation and excrete them. It is clear that in kidney injury situations, accumulation of these molecules and peptides leads to increased concentration of substances in blood and initiates immune responses with deleterious effects in distant organs. In addition, epithelial tubular cells are active to producing a variety of inflammatory mediators with presenting circulatory antigens and promoting the activation of leukocytes that passing through the kidney via this rich circulation.1 Now it is known that renal epithelial cells up regulate and secrete some chemokines and cytokines such as nuclear factor-κB (NF-κB) in injured situations, which can initiate the inflammatory cascade in other organs.2, 3
Acute kidney injury (AKI), also known as acute renal failure, is a common clinical disorder resulting from some conditions such as renal ischemia reperfusion injury with an abrupt loss of kidney function and decline in renal filtration fraction.4, 5, 6 The incidence of AKI varies about 5%–7% in hospitalized patients and it seems that this ratio is rising every year.7 Despite recent advances in the treatment of AKI, this disorder still has a high mortality and morbidity rates in approximately 50% hospitalized patients, presumably due to the unchanged dysfunction of other organs.8 Recent studies have found an association between kidney and remote organs dysfunction.9, 10 In most cases kidney disease directly or indirectly affects pulmonary functions and causes the lungs to be recognized as one of the most affected distant organs of kidney injury.11 Respiratory complications are mostly associated with renal failure, and conversely AKI is a common incidence in mechanically ventilated patients.12
This crosstalk involves a complex interaction between many of biochemical, cellular and tissue specific factors that excite remote pro-inflammatory and pro-apoptotic signaling.13, 14 The innate immune pathways were mostly mediated through production of oxygen free radicals, secretion of inflammatory cytokines and recruitment of polymorphonuclear cells.13, 15 Impaired renal filtration leads to elevated trans-capillary filtration pressure gradient and promotes tissue edema.16 Edema especially has serious consequences in the lungs because pulmonary edema impairs gas exchange and can lead to potentially life-threatening condition.17 Pulmonary failure can develop to acute lung injury (ALI) and eventually respiratory distress syndrome with a high mortality rate. The mortality rate of ALI alone is 30%–40%, but the rate rises to 80% in combination with AKI.11, 18, 19 Therefore, at least partial causes of the high morbidity and mortality rate of AKI derive from extrarenal complications, usually related to pulmonary dysfunction,20 which shows particular importance of extrarenal organs complications and requires knowledge of link between lung and kidney in determining therapeutic strategies to decrease the mortality rate in critically ill patients. Unfortunately, little is known about the potential interactions between these tissues in critically ill patients. In this review we summarize some potential mechanisms, diagnostic biomarkers and treatments involved in the acute respiratory distress syndrome (ARDS) after renal failure.
Pathophysiological interactions of kidney injury and ARDS
Edema
One of the most effects of AKI on pulmonary system is through the water imbalance. Pulmonary fluid and electrolyte transporters change after AKI. Sodium ATPase pump and epithelial sodium channel (ENaC) promote sodium absorption from the alveolar cavity into the alveolar epithelium cells. Then, water passively follows sodium out of the alveoli. Studies have shown that renal failure can down regulate the epithelial salt-water transporters such as ENaC, sodium-potassium ATPase and aquaporin-5 in the lung, which all contribute to high pulmonary vascular permeability and low alveolar fluid clearance.21, 22, 23, 24 This type of edema is a consequences of following disorders: water-sodium retention induced by renal injury; increased hydrostatic pulmonary capillary pressures and changed Starling's forces; loss of membrane integrity in capillary endothelial and alveoli epithelial; leakage of plasma protein and alveolar fluid accumulation.25 Because the lung contains many blood vessels, it is the most vulnerable organ to injury.26 Pulmonary edema patients have prolonged hospital stays, mechanical ventilation, and higher rates of pneumonia. Renal injury-induced water retention results in decreased pulmonary compliance and increased respiratory work in patients.27 These conditions lead to impaired gas exchange, which can be severe refractory arterial hypoxemia and life-threatening.28 Any intervention to reduce pulmonary edema can have a significant effect in improving patients' health.
Pulmonary edema has many plasma proteins including proteolytic enzymes, proteins, fibrinogen and fibrin in its contents, which can lead to destruction of the surfactant proteins. The damage of alveolar epithelial cells caused by inflammatory mediators can have additional effects in the destruction and decrease of surfactant. Although over-load volume of renal failure has an important role in the onset of ALI but evidence indicates that lung damage may occur even in absence of positive fluid balance.24 On the other hand, it seems that the uremia is responsible for effects of renal injury on the lung's salt and water transporters.29, 30
Cytokines
The harmful effects of AKI on the lung function could relate to the loss of normal balance of immune, inflammatory and soluble mediator metabolism.31 The kidney plays a key role in cytokines metabolism and clearance. Impaired kidney function is associated with cytokine imbalance (both production and elimination) in the circulation. It revealed that an important pathway of lung injury subsequent kidney injury could arise from cytokine dysregulation in the kidney, with further activation of the lung's indigenous immune cells and respiratory complications.12 Additionally there is a massive system of vessels in the lungs that accelerate lung deposition of multiple inflammatory mediators. The up-regulation of pro-inflammatory genes and inflammatory cytokines after AKI have important effects on the onset and progression of ALI.32 Animal experiments have shown that AKI causes the activation of proinflammatory and anti-inflammatory mediator's gene in the lung.29 The products of these proinflammatory genes such as Cd14, lipocalin-2, chemokine ligand-2 (CXCL2), and IL-6 can be released into circulation and initiate inflammation cascade in pulmonary.33
In addition, inflammatory cytokines especially interleukins (IL-6, IL-8, IL-1β), tumor necrosis factor α (TNF-α), macrophage inflammatory protein 2, amyloid protein A are the main mediators involved in the progress of distant organs injury including lung failure after AKI.12, 29, 33 NF-κB is a pro-inflammatory transcription factor that leads to gene expression of inflammatory proteins, including cytokines, chemokines and adhesion molecules.34
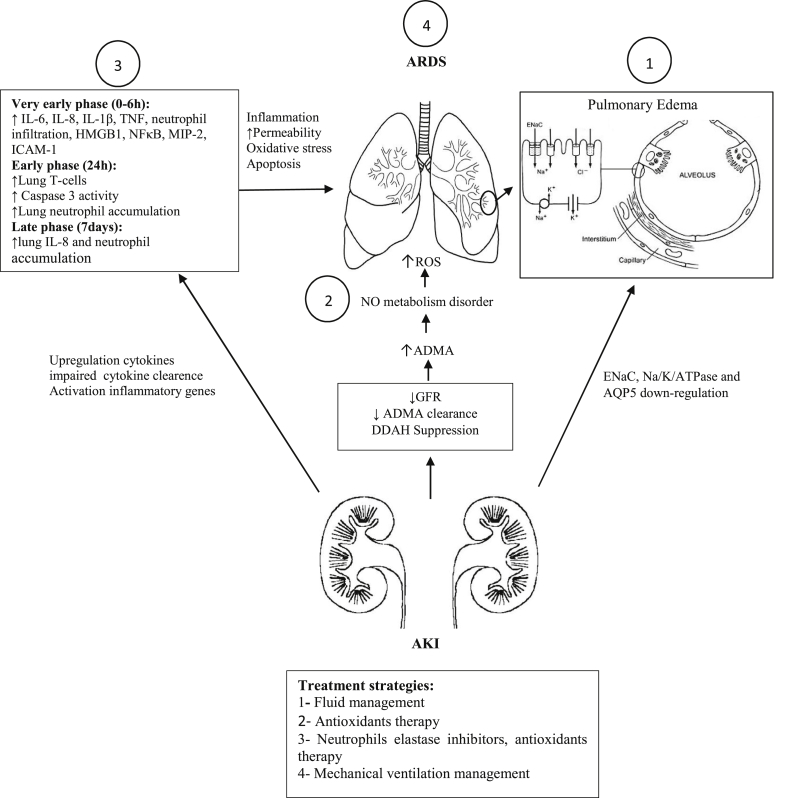
The systematic inflammatory reactions, accumulated toxic metabolites after AKI, and the decrease of omission and inactivation of the inflammatory mediators through kidneys, cause increase of mediators in the plasma.26 These mediators can change pulmonary vascular permeability which exacerbates edema, leukocyte infiltration and respiratory disorders.35, 36, 37, 38, 39, 40 IL-6 seems to be a patient mortality factor in AKI due to its particular role in the initiation and extension of the inflammatory process.12, 39 Recently, Klein et al12 demonstrated that IL-6 knockout mice models have less neutrophil infiltration, myeloperoxidase activity and capillary permeation resulting in lower pulmonary edema. TNF-α also is a vital cytokine in mediating ALI. It persuades the pulmonary endothelial cells activation, white blood cells migration, granulocyte degranulation, reactive oxygen species stimulation and capillary leakage.41 Furthermore, TNF-α interacts with multiple cytokines which can induce extensive effects. For example TNF-α increases the genesis of IL-6.42 We can classify the release of different cytokines in AKI-induced ARDS as diagnostic biomarkers in time variant occurrence phases (Fig. 1).
Fig. 1.
The effects of AKI on lung dysfunction. AKI caused lung inflammatory responses and apoptosis through releasing many inflammatory mediators and cytokines. These mediators can be used as diagnostic markers in three time phases: very early (within 0–6 h), early (24 h) and late (7 d). Neutrophil accumulation and trafficking occurs following inflammation, cytokines and integrins activation such as IL-6 and ICAM-1. On the other hand, renal dysfunction after AKI leading to decreased GFR and ADMA clearance with NO metabolism disorder and reactive oxygen production. Pulmonary edema is often caused by down-regulation of ENaC, Na/K/ATPase and AQP5. As a result, all events lead to increased permeability and edema, oxidative stress and apoptosis in the lung that finally caused ARDS.
Abbreviations: GFR, glomerular filtration rate; AKI, acute kidney injury; ICAM-1, intercellular adhesion molecule 1; ADMA, asymmetric dimethyl arginine; ENaC, epithelial sodium channel; AQP5, aquaporin 5; HMGB1, high-mobility group protein B1; MIP-2, macrophage inflammatory protein 2; NFkB, nuclear factor kB; TNF, tumor necrosis factor.
Neutrophil trafficking
Neutrophils are the first immune cells to arrive at the site of injury or inflammation. After activation, neutrophils inflow from the vascular endothelial cells to the interstitium and into the alveolar space. Recruitment of neutrophils into the lung is one of the key events in the development of ARDS.43 Alveolar capillaries are the main site of sequestration and margination of neutrophils.44 Lung capillary network consist of large number of segments with about 40% equal to, or smaller diameter than the neutrophils.45 Almost 50% of the circulating leukocyte population can be segregated within the pulmonary vasculature.46, 47, 48 Pulmonary neutrophil sequestration is an early event that occurs in pathologic lung inflammation.49 Apoptotic events and inflammatory mediators especially the cytokines IL-6 and IL-8 are responsible of the leukocyte recruitment during the inflammatory response of AKI.50 Moreover, cytokines and chemokines cause integrins activation leading to adhesion of neutrophils on the endothelium.51 It appears that β2-integrins have particular role in neutrophil recruitment.52
Neutrophils margination to vascular endothelium participates in microvascular plug, vascular congestion and damaging by releasing reactive oxygen species and potent proteolytic enzymes.51 Neutrophils can also release a variety of cytokines including interferon (IFN)-γ,53 IL-4,54 IL-6,55 IL-10,56 and TNF-α.57
It appears that neutrophils and neutrophil elastase, a serine protease which is available in the granules of the neutrophil, have important roles in endothelial injury and increased vascular permeability in ARDS.58
Oxidative stress
Oxidative stress and its systemic consequences likely play a significant role in AKI-induced lung injury. The increased lung tissue levels of malondialdehyde (MDA) (a marker of lipid peroxidation) have been observed in rats with AKI.26, 59
There are three main sources of oxidative stress: 1) Activation of neutrophils in the pulmonary circulation causes the release of large amounts free radicals and reactive oxygen species60; 2) Accumulation of activated macrophages to injured tissue can induce cell death by releasing reactive oxygen species; 3) Last source of oxidative stress in ARDS patients is availability of high levels of oxygen employed during ventilator therapy. It seems that antioxidant activity and potency also decreased in these patients.61 Glutathione is an important antioxidant in the lung that decreases in patients with ARDS.62 Metnitz and colleagues63 showed that plasma levels of alpha-tocopherol, vitamin C, beta-carotene, and selenium were reduced in ARDS patients. These events lead to the increased production of oxidants, creating an imbalance between antioxidants and oxidants which will lead to the pathways of cell death. Inflammation condition in lung injury is a suitable opportunity for free radicals to overwhelm the endogenous antioxidants.
Inflammatory factors following AKI activate oxidative stress and reactive oxygen species production that can lead to ALI by several mechanisms including: lipid peroxidation, direct oxidative damage and mutations in DNA, changes in cellular protein activity by proteins and enzymes oxidation, alteration in genomic transcription and direct surfactant damage.64 Cellular DNA damage inhibits protein syntheses that are involved in cell growth, genes encoding antioxidant enzymes and cell repair.65
Apoptosis
AKI activates variants lung apoptosis-related genes including tumor necrosis factor receptor 1 (TNFR1) and programmed cell death.
Tumor necrosis factor receptor 1 (TNFR1)-mediated programmed cell death66 and lung microvascular barrier dysfunction67 have been identified prominent factors in mediating lung dysfunction through endothelial cell apoptosis. Endothelial cell apoptosis has deleterious effects on solute transport across the vascular membrane. Impaired endothelial barrier function has a key role in increased vascular permeability and inflammation.68 There was increased lung vascular permeability at 24 and 48 h post ischemia in a rat model of bilateral renal ischemia reperfusion injury, quantified by leakage of labeled albumin outside the vascular space.23, 69 Pulmonary cellular apoptosis may also contribute to ARDS.
AKI also leads to an increase in lung caspases. Administration of caspase inhibitors reduces lung injury following acute renal failure.15
Asymmetric dimethyl arginine
NO metabolism disorder due to renal failure makes the lungs more sensitive to injury. The mechanism underlying NO dysregulation is not completely clear, but it appears that asymmetric dimethyl arginine (ADMA), an inhibitor of endothelial NO synthase (eNOS), plays a significant role. ADMA shifts NO metabolism toward the production of free radicals and lung damage.70 ADMA is in part excreted by renal excretion.71 Reduced glomerular filtration fraction of ADMA in renal failure is associated with slight increases of ADMA plasma levels and impaired pulmonary vascular vasodilation.72 The high-level clearance process of ADMA is carried out by dimethylarginine dimethylaminohydrolase (DDAH), enzyme involved in the degradation of the ADMA. It seems that DDAH is suppressed in acute renal injury, leading to accumulation of ADMA in plasma and tissues.73
Diagnostic biomarkers
Some mediators and indicators indicate that lung injury after AKI can be classified into 3 phases according to the time of occurrence, as follows:
-
1)
Very early phase (0–6 h): Pulmonary edema and lung neutrophil accumulation occur very early with increased serum proinflammatory cytokines. Increased serum IL-6 (not in local lung), within 2 h in patients with AKI leads to increased lung chemokine (C-X-C motif) ligand 1 (CXCL1) such as IL-8 production and neutrophil infiltration.36, 74 Therefore, increased serum and lung IL-8 at 2 h after AKI can predict lung injury.36 Tumor necrosis factor (TNF) is another marker that induces TNFR1-mediated pulmonary apoptosis within 2 h after ischemic AKI.66 Increased serum high-mobility group protein B1 (HMGB1), an agonist of TLR-4, within 6 h after AKI75 and lung markers such as nuclear factor κB (NFκB),76 macrophage inflammatory protein 2 (MIP-2),29 intercellular adhesion molecule 1 (ICAM-1)77 and IL-1β75 are associated with lung injury after AKI.
-
2)
Early phase (24 h): lung T-cell accumulation via increased lung endothelial apoptosis with caspase 3 activity and lung TUNEL staining is responsible of continuing pulmonary edema and lung neutrophil accumulation within 24 h after AKI onset.14, 67
-
3)
Late phase (7 days): increased lung IL-8 and lung neutrophil accumulation indicate lung injury occurrence in AKI patients.78
Treatment strategies
Mechanical ventilation management
Protective ventilation term means sufficient oxygenation of the blood and carbon dioxide elimination to avoid from over distension, barotrauma, atelectasis, hemodynamic impairment, and patient-ventilator asynchrony. The choice of proper ventilation strategy is capable of preventing the progression of the lung disease and its outcomes. Incorrect mechanical ventilation methods can be associated with more damaging effects and increased mortality that named ventilator-associated lung injury. The use of adequate levels of tidal volume 6 ml/kg predicted body weight (PBW), plateau pressure kept below 30 cm H2O, inspiratory oxygen concentration (FiO2) as low as possible, and a permissive hypercapnia to a pH level of 7.2 are more successful strategies in protective ventilation to ARDS therapy.79, 80
This protective ventilation has important consequences in AKI and ARDS patients. It can prevent the development of AKI by creating mild hypercapnic that is well tolerated. Mild hypercapnic acidosis has shown anti-inflammatory and cytoprotective effects.81, 82 Inactivation of calcium channels and vasodilation of vessels, decrease in NF-kappaB production and cytokines releasing are involved in protective effects of hypercapnic acidosis.83, 84, 85
Fluid management
Fluid accumulation has an adverse effect on recognition of AKI due to dilution of creatinine in body compartments. Therefore, an increase of 0.3 mg/dl or change more than 50% within 48 h in creatinine concentration must be considered as a diagnostic criterion of AKI.86 Fluid management in ARDS with AKI is complicated because of two aspects: first, liberal fluid therapy is required for kidney perfusion and second, restriction of fluids with a diuretic drug can limit the amount of lung edema, reduce pulmonary capillary pressure, central venous pressure and eventually decrease mortality.87, 88 But according to a study that compared two fluid protocol therapies (liberal and conservative) with 1000 patients in 2006, showed the conservative fluid therapy using diuretics has better outcomes, including shorter duration of mechanical ventilation and ICU stays.89
Neutrophils elastase inhibitors
Neutrophil elastase is a serine protease secreted from neutrophils in inflammation and has a significant role in pathogenesis of AKI-induced ALI.90 It seems that neutrophils elastase inhibitors such as Sivelestat are able to inhibit the progression of ARDS and AKI.91, 92 Sivelestat decreases neutrophil infiltration and cytokines expression in the lung92 and has improve outcome in multiple organ failure.93
Antioxidants therapy
Administration of antioxidants has beneficial effects in ARDS patients with AKI. Antioxidant vitamins and trace elements such as vitamins A, C, and E, selenium and zinc have radical scavengers and antioxidant activities.94 However, the use of high doses of antioxidant vitamins and trace elements is not recommended in patients with nephrogenic ARDS due to renal impairment.
Vitamin C reacts directly with free radicals and can restore antioxidant property of oxidized vitamin E.95 Zinc does not interact directly with free radicals but it can increase the activity of antioxidant enzymes such as superoxide dismutase (SOD).96 In addition, zinc inhibits pro-oxidant enzymes such as the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, inducible NOS (iNOS).97
Selenium is a potent antioxidant because it can bind to hydroperoxides (H2O2) with more affinity than catalase.98 SOD or synzyme mimics are catalytic drugs with potent anti-inflammatory and ROS detoxification properties.99 It seems that SOD mimics inhibit neutrophil-mediated injuries.99
Diseases affecting both lungs and kidneys
There are several “pulmonary renal syndromes” that affect both the kidneys and the lungs.100, 101 These disorders are often associated with hemoptysis from diffuse alveolar hemorrhage along with renal insufficiency from either acute glomerulonephritis or other vasculitis.57, 58 Three of these most familiar diseases are Wegener's granulomatosis, systemic lupus erythematosus, and Goodpasture's syndrome.102 Some of the known diseases with both pulmonary and renal manifestations were listed as follows:
Wegener's granulomatosis;
Microscopic polyangiitis;
Mixed cryoglobulinemia;
Henoch-Schonlein purpura;
Immune complex glomerulonephritis;
Pauci-immune glomerulonephritis;
Systemic lupus erythematosus;
Goodpasture syndrome;
Thrombotic thrombocytopenic purpura;
Allergic granulomatous angiitis (Churg–Strauss syndrome).
Conclusion
Pulmonary dysfunction is a common complication in patients with AKI that contributes to increasing the mortality rate. The kidney-lung crosstalk in AKI and ARDS is a consequence of complex biological process which leads to dysregulation of cytokines/mediators and apoptotic signaling pathways. This review summarized the most important various aspects of pathophysiology, diagnostic and treatment involved in lung injury associated with AKI. Better understanding this relation can be a gateway to novel therapeutic strategies against AKI and decrease high mortality rate during AKI-related pulmonary failure.
Acknowledgements
We thank Professor Mehdi Nematbakhsh for his time and effort to review our manuscript.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.de Haij S., Woltman A.M., Bakker A.C. Production of inflammatory mediators by renal epithelial cells is insensitive to glucocorticoids. Br J Pharmacol. 2002;137:197–204. doi: 10.1038/sj.bjp.0704866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton C.J., Combe C., Walls J. Secretion of chemokines and cytokines by human tubular epithelial cells in response to proteins. Nephrol Dial Transpl. 1999;14:2628–2633. doi: 10.1093/ndt/14.11.2628. [DOI] [PubMed] [Google Scholar]
- 3.Markó L., Vigolo E., Hinze C. Tubular epithelial NF-κB activity regulates ischemic AKI. J Am Soc Nephrol. 2016;27:2658–2669. doi: 10.1681/ASN.2015070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellomo R., Kellum J.A., Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 5.Malek M., Nematbakhsh M. The preventive effects of diminazene aceturate in renal ischemia/reperfusion injury in male and female rats. Adv Prev Med. 2014;2014 doi: 10.1155/2014/740647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malek M., Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Ren Inj Prev. 2015;4:20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chertow G.M., Burdick E., Honour M. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 8.Ympa Y.P., Sakr Y., Reinhart K. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med. 2005;118:827–832. doi: 10.1016/j.amjmed.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 9.Azarkish F., Nematbakhsh M., Fazilati M. N-acetylcysteine prevents kidney and lung disturbances in renal ischemia/reperfusion injury in rat. Int J Prev Med. 2013;1:1139–1146. [PMC free article] [PubMed] [Google Scholar]
- 10.Malek M., Maleki M. Protective effect of gastric distension preconditioning on renal ischemia/reperfusion injury in rats. Indian J Nephrol. 2017 doi: 10.4103/ijn.IJN_342_16. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paladino J.D., Hotchkiss J.R., Rabb H. Acute kidney injury and lung dysfunction: a paradigm for remote organ effects of kidney disease? Microvasc Res. 2009;77:8–12. doi: 10.1016/j.mvr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein C.L., Hoke T.S., Fang W.F. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74:901–909. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 13.Hassoun H.T., Grigoryev D.N., Lie M.L. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Ren Physiol. 2007;293:F30–F40. doi: 10.1152/ajprenal.00023.2007. [DOI] [PubMed] [Google Scholar]
- 14.Lie M.L., White L.E., Santora R.J. Lung T lymphocyte trafficking and activation during ischemic acute kidney injury. J Immunol. 2012;189:2843–2851. doi: 10.4049/jimmunol.1103254. [DOI] [PubMed] [Google Scholar]
- 15.Hassoun H.T., Lie M.L., Grigoryev D.N. Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am J Physiol Ren Physiol. 2009;297:F125–F137. doi: 10.1152/ajprenal.90666.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vande Walle J.G., Donckerwolcke R. Pathogenesis of edema formation in the nephrotic syndrome. Pediatr Nephrol. 2001;16:283–293. doi: 10.1007/s004670000512. [DOI] [PubMed] [Google Scholar]
- 17.Bagshaw S.M., Brophy P.D., Cruz D. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit care. 2008;12:169. doi: 10.1186/cc6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson T.A., Caldwell E.S., Curtis J.R. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354–360. doi: 10.1001/jama.281.4.354. [DOI] [PubMed] [Google Scholar]
- 19.Mehta R.L., Pascual M.T., Gruta C.G. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–1357. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg R., Dennen P. Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis. 2008;15:297–307. doi: 10.1053/j.ackd.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Borok Z., Verkman A.S. Lung edema clearance: 20 years of progress: invited review: role of aquaporin water channels in fluid transport in lung and airways. J Appl Physiol (1985) 2002;93:2199–2206. doi: 10.1152/japplphysiol.01171.2001. [DOI] [PubMed] [Google Scholar]
- 22.Campanholle G., Landgraf R.G., Gonçalves G.M. Lung inflammation is induced by renal ischemia and reperfusion injury as part of the systemic inflammatory syndrome. Inflamm Res. 2010;59:861–869. doi: 10.1007/s00011-010-0198-0. [DOI] [PubMed] [Google Scholar]
- 23.Kramer A.A., Postler G., Salhab K.F. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55:2362–2367. doi: 10.1046/j.1523-1755.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- 24.Rabb H., Wang Z., Nemoto T. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int. 2003;63(2):600–606. doi: 10.1046/j.1523-1755.2003.00753.x. [DOI] [PubMed] [Google Scholar]
- 25.Basu R.K., Wheeler D. Effects of ischemic acute kidney injury on lung water balance: nephrogenic pulmonary edema? Pulm Med. 2011;2011 doi: 10.1155/2011/414253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma T., Liu X.W., Liu Z. Function of the p38MAPK-HSP27 pathway in rat lung injury induced by acute ischemic kidney injury. Biomed Res Int. 2013;2013 doi: 10.1155/2013/981235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin G.S. Fluid management in acute lung injury and ARDS. Neth J Crit Care. 2012;16(1) https://www.njcc.nl/sites/nvic.nl/files/NJCC%2001%20review-Martin.pdf [Google Scholar]
- 28.ARDS Definition Task Force, Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 29.Hoke T.S., Douglas I.S., Klein C.L. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18(1):155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 30.Kim D.J., Park S.H., Sheen M.R. Comparison of experimental lung injury from acute renal failure with injury due to sepsis. Respiration. 2006;73:815–824. doi: 10.1159/000095588. [DOI] [PubMed] [Google Scholar]
- 31.Ko G.J., Rabb H., Hassoun H.T. Kidney-lung crosstalk in the critically ill patient. Blood Purif. 2009;28:75–83. doi: 10.1159/000218087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemay S., Rabb H., Postler G. Prominent and sustained up-regulation of gp130-signaling cytokines and of the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation. 2000;69:959–963. doi: 10.1097/00007890-200003150-00049. [DOI] [PubMed] [Google Scholar]
- 33.Grigoryev D.N., Liu M., Hassoun H.T. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19:547–558. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J.I., Burckart G.J. Nuclear factor kappa B: important transcription factor and therapeutic target. J Clin Pharmacol. 1998;38:981–993. doi: 10.1177/009127009803801101. [DOI] [PubMed] [Google Scholar]
- 35.Chawla L.S., Seneff M.G., Nelson D.R. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol. 2007;2:22–30. doi: 10.2215/CJN.02510706. [DOI] [PubMed] [Google Scholar]
- 36.Liu K.D., Altmann C., Smits G. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care. 2009;13:R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liangos O., Kolyada A., Tighiouart H. Interleukin-8 and acute kidney injury following cardiopulmonary bypass: a prospective cohort study. Nephron Clin Pract. 2009;113:c148–c154. doi: 10.1159/000232595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon O., Molitoris B.A., Pescovitz M. Urinary actin, interleukin-6, and interleukin-8 may predict sustained ARF after ischemic injury in renal allografts. Am J Kidney Dis. 2003;41:1074–1087. doi: 10.1016/s0272-6386(03)00206-3. [DOI] [PubMed] [Google Scholar]
- 39.Simmons E.M., Himmelfarb J., Sezer M.T. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–1365. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 40.Meduri G.U., Headley S., Kohler G. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS plasma IL-1β and IL-6 levels are consistent and efficient predictors of outcome over time. Chest J. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay S., Hoidal J.R., Mukherjee T.K. Role of TNFalpha in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurokouchi K., Kambe F., Yasukawa K. TNF-α increases expression of IL-6 and ICAM-1 genes through activation of NF-κB in osteoblast-like ROS17/2.8 cells. J Bone Min Res. 1998;13:1290–1299. doi: 10.1359/jbmr.1998.13.8.1290. [DOI] [PubMed] [Google Scholar]
- 43.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 44.Doyle N.A., Bhagwan S.D., Meek B.B. Neutrophil margination, sequestration, and emigration in the lungs of L-selectin-deficient mice. J Clin Invest. 1997;99:526–533. doi: 10.1172/JCI119189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bathe M., Shirai A., Doerschuk C.M. Neutrophil transit times through pulmonary capillaries: the effects of capillary geometry and fMLP-stimulation. Biophys J. 2002;83:1917–1933. doi: 10.1016/S0006-3495(02)73955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rimensberger P.C., Fedorko L., Cutz E. Attenuation of ventilator-induced acute lung injury in an animal model by inhibition of neutrophil adhesion by leumedins (NPC 15669) Crit Care Med. 1998;26:548–555. doi: 10.1097/00003246-199803000-00030. [DOI] [PubMed] [Google Scholar]
- 47.Wiggs B.R., English D., Quinlan W.M. Contributions of capillary pathway size and neutrophil deformability to neutrophil transit through rabbit lungs. J Appl Physiol. 1994;77:463–470. doi: 10.1152/jappl.1994.77.1.463. [DOI] [PubMed] [Google Scholar]
- 48.Doerschuk C.M., Beyers N., Coxson H.O. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J Appl Physiol (1985) 1993;74:3040–3045. doi: 10.1152/jappl.1993.74.6.3040. [DOI] [PubMed] [Google Scholar]
- 49.Doerschuk C.M. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71–88. [PubMed] [Google Scholar]
- 50.Awad A.S., Rouse M., Huang L. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009;75:689–698. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou X., Dai Q., Huang X. Neutrophils in acute lung injury. Front Biosci (Landmark Ed) 2011;17:2278–2283. doi: 10.2741/4051. [DOI] [PubMed] [Google Scholar]
- 52.Kim S., Schein A.J., Nadel J.A. E-cadherin promotes EGFR-mediated cell differentiation and MUC5AC mucin expression in cultured human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1049–L1060. doi: 10.1152/ajplung.00388.2004. [DOI] [PubMed] [Google Scholar]
- 53.Li L., Huang L., Sung S.S. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007;178:5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 54.Brandt E., Woerly G., Younes A.B. IL-4 production by human polymorphonuclear neutrophils. J Leukoc Biol. 2000;68:125–130. [PubMed] [Google Scholar]
- 55.Retini C., Vecchiarelli A., Monari C. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect Immun. 1996;64:2897–2903. doi: 10.1128/iai.64.8.2897-2903.1996. 174164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffman S.J., Polack F.P., Hauer D.A. Measles virus infection of rhesus macaques affects neutrophil expression of IL-12 and IL-10. Viral Immunol. 2003;16:369–379. doi: 10.1089/088282403322396163. [DOI] [PubMed] [Google Scholar]
- 57.Ethuin F., Gérard B., Benna J.E. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–1371. doi: 10.1038/labinvest.3700148. [DOI] [PubMed] [Google Scholar]
- 58.Lee W.L., Downey G.P. Leukocyte elastase: physiological functions and role in acute lung injury. Am J Respir Crit Care Med. 2001;164:896–904. doi: 10.1164/ajrccm.164.5.2103040. [DOI] [PubMed] [Google Scholar]
- 59.Steinberg J., Halter J., Schiller H. Chemically modified tetracycline prevents the development of septic shock and acute respiratory distress syndrome in a clinically applicable porcine model. Shock. 2005;24:348–356. doi: 10.1097/01.shk.0000180619.06317.2c. [DOI] [PubMed] [Google Scholar]
- 60.Grammes J., Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sabarirajan J., Vijayaraj P., Nachiappan V. Induction of acute respiratory distress syndrome in rats by lipopolysaccharide and its effect on oxidative stress and antioxidant status in lung. Indian J Biochem Biophys. 2010;47:278–284. [PubMed] [Google Scholar]
- 62.Bernal M.E., Varon J., Acosta P. Oxidative stress in critical care medicine. Int J Clin Pract. 2010;64(11):1480–1488. doi: 10.1111/j.1742-1241.2010.02506.x. [DOI] [PubMed] [Google Scholar]
- 63.Metnitz P.G., Bartens C., Fischer M. Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Med. 1999;25:180–185. doi: 10.1007/s001340050813. [DOI] [PubMed] [Google Scholar]
- 64.Chow C.W., Herrera Abreu M.T., Suzuki T. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003;29:427–431. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- 65.Clement A., Housset B. Role of free radicals in airways injury. In: Chretien J., Dusser D., editors. Environmental impact on the airways. New York; Dekker: 1996. pp. 355–379. [Google Scholar]
- 66.White L.E., Santora R.J., Cui Y. TNFR1-dependent pulmonary apoptosis during ischemic acute kidney injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L449–L459. doi: 10.1152/ajplung.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White L.E., Cui Y., Shelak C.M. Lung endothelial cell apoptosis during ischemic acute kidney injury. Shock. 2012;38:320–327. doi: 10.1097/SHK.0b013e31826359d0. [DOI] [PubMed] [Google Scholar]
- 68.Imai Y., Parodo J., Kajikawa O. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 69.Levy E.M., Viscoli C.M., Horwitz R.I. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–1494. [PubMed] [Google Scholar]
- 70.Wells S.M., Holian A. Asymmetric dimethylarginine induces oxidative and nitrosative stress in murine lung epithelial cells. Am J Respir Cell Mol Biol. 2007;36:520–528. doi: 10.1165/rcmb.2006-0302SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kimoto M., Whitley G.S.J., Tsuji H. Detection of NG, NG-dimethylarginine dimethylaminohydrolase in human tissues using a monoclonal antibody. J Biochem. 1995;117:237–238. doi: 10.1093/jb/117.2.237. [DOI] [PubMed] [Google Scholar]
- 72.Vallance P., Leone A., Calver A. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 73.Nakayama Y., Ueda S., Yamagishi S. Asymmetric dimethylarginine accumulates in the kidney during ischemia/reperfusion injury. Kidney Int. 2014;85:570–578. doi: 10.1038/ki.2013.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahuja N., Andres-Hernando A., Altmann C. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am J Physiol Ren Physiol. 2012;303:F864–F872. doi: 10.1152/ajprenal.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doi K., Ishizu T., Tsukamoto-Sumida M. The high-mobility group protein B1-Toll-like receptor 4 pathway contributes to the acute lung injury induced by bilateral nephrectomy. Kidney Int. 2014;86:316–326. doi: 10.1038/ki.2014.62. [DOI] [PubMed] [Google Scholar]
- 76.Deng J., Hu X., Yuen P.S. Alpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/reperfusion. Am J Respir Crit Care Med. 2004;169:749–756. doi: 10.1164/rccm.200303-372OC. [DOI] [PubMed] [Google Scholar]
- 77.Seekamp A., Mulligan M.S., Till G.O. Role of beta 2 integrins and ICAM-1 in lung injury following ischemia-reperfusion of rat hind limbs. Am J Pathol. 1993;143:464–472. [PMC free article] [PubMed] [Google Scholar]
- 78.Faubel S., Edelstein C.L. Mechanisms and mediators of lung injury after acute kidney injury. Nat Rev Nephrol. 2016;12:48–60. doi: 10.1038/nrneph.2015.158. [DOI] [PubMed] [Google Scholar]
- 79.Acute Respiratory Distress Syndrome Network. Brower R.G., Matthay M.A., Morris A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 80.Hager D.N., Krishnan J.A., Hayden D.L. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Res Crit Care Med. 2005;172:1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ismaiel N.M., Henzler D. Effects of hypercapnia and hypercapnic acidosis on attenuation of ventilator-associated lung injury. Minerva Anestesiol. 2011;77:723–733. [PubMed] [Google Scholar]
- 82.Peltekova V., Engelberts D., Otulakowski G. Hypercapnic acidosis in ventilator-induced lung injury. Intensive Care Med. 2010;36:869–878. doi: 10.1007/s00134-010-1787-7. [DOI] [PubMed] [Google Scholar]
- 83.Coakley R.J., Taggart C., Greene C. Ambient pCO2 modulates intracellular pH, intracellular oxidant generation, and interleukin-8 secretion in human neutrophils. J Leukoc Biol. 2002;71:603–610. [PubMed] [Google Scholar]
- 84.Laffey J.G., Tanaka M., Engelberts D. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med. 2000;162:2287–2294. doi: 10.1164/ajrccm.162.6.2003066. [DOI] [PubMed] [Google Scholar]
- 85.Tak P.P., Gerlag D.M., Aupperle K.R. Inhibitor of nuclear factor κB kinase β is a key regulator of synovial inflammation. Arthritis Rheum. 2001;44:1897–1907. doi: 10.1002/1529-0131(200108)44:8<1897::AID-ART328>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 86.Liu K.D., Thompson B.T., Ancukiewicz M. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011;39:2665–2671. doi: 10.1097/CCM.0b013e318228234b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roch A., Guervilly C., Papazian L. Fluid management in acute lung injury and ards. Ann Intensive Care. 2011;1:16. doi: 10.1186/2110-5820-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grams M.E., Estrella M.M., Coresh J. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6:966–973. doi: 10.2215/CJN.08781010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann H.P., Wheeler A.P. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 90.Zeiher B.G., Matsuoka S., Kawabata K. Neutrophil elastase and acute lung injury: prospects for sivelestat and other neutrophil elastase inhibitors as therapeutics. Crit Care Med. 2002;30:S281–S287. doi: 10.1097/00003246-200205001-00018. [DOI] [PubMed] [Google Scholar]
- 91.Okayama N., Kakihana Y., Setoguchi D. Clinical effects of a neutrophil elastase inhibitor, sivelestat, in patients with acute respiratory distress syndrome. J Anesth. 2006;20:6–10. doi: 10.1007/s00540-005-0362-9. [DOI] [PubMed] [Google Scholar]
- 92.Ishii T., Doi K., Okamoto K. Neutrophil elastase contributes to acute lung injury induced by bilateral nephrectomy. Am J Pathol. 2010;177:1665–1673. doi: 10.2353/ajpath.2010.090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoshi K., Kurosawa S., Kato M. Sivelestat, a neutrophil elastase inhibitor, reduces mortality rate of critically ill patients. Tohoku J Exp Med. 2005;207:143–148. doi: 10.1620/tjem.207.143. [DOI] [PubMed] [Google Scholar]
- 94.Koekkoek W.K., van Zanten A.R. Antioxidant vitamins and trace elements in critical illness. Nutr Clin Pract. 2016;31:457–474. doi: 10.1177/0884533616653832. [DOI] [PubMed] [Google Scholar]
- 95.Berger M.M., Oudemans-van Straaten H.M. Vitamin C supplementation in the critically ill patient. Curr Opin Clin Nutr Metab Care. 2015;18:193–201. doi: 10.1097/MCO.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 96.Kloubert V., Rink L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Func. 2015;6:3195–3204. doi: 10.1039/c5fo00630a. [DOI] [PubMed] [Google Scholar]
- 97.Cruz K.J., de Oliveira A.R., Marreiro Ddo N. Antioxidant role of zinc in diabetes mellitus. World J Diabetes. 2015;6:333–337. doi: 10.4239/wjd.v6.i2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venardos K.M., Perkins A., Headrick J. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Curr Med Chem. 2007;14:1539–1549. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- 99.Cuzzocrea S., Riley D.P., Caputi A.P. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 100.Pierson D.J. Respiratory considerations in the patient with renal failure. Respir Care. 2006;51:413–422. [PubMed] [Google Scholar]
- 101.Rodriguez W., Hanania N., Guy E. Pulmonary-renal syndromes in the intensive care unit. Crit Care Clin. 2002;18:881–895. doi: 10.1016/s0749-0704(02)00029-5. [DOI] [PubMed] [Google Scholar]
- 102.von Vigier R.O., Trummler S.A., Laux-End R. Pulmonary renal syndrome in childhood: a report of twenty-one cases and a review of the literature. Pediatr Pulmonol. 2000;29:382–388. doi: 10.1002/(sici)1099-0496(200005)29:5<382::aid-ppul7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]