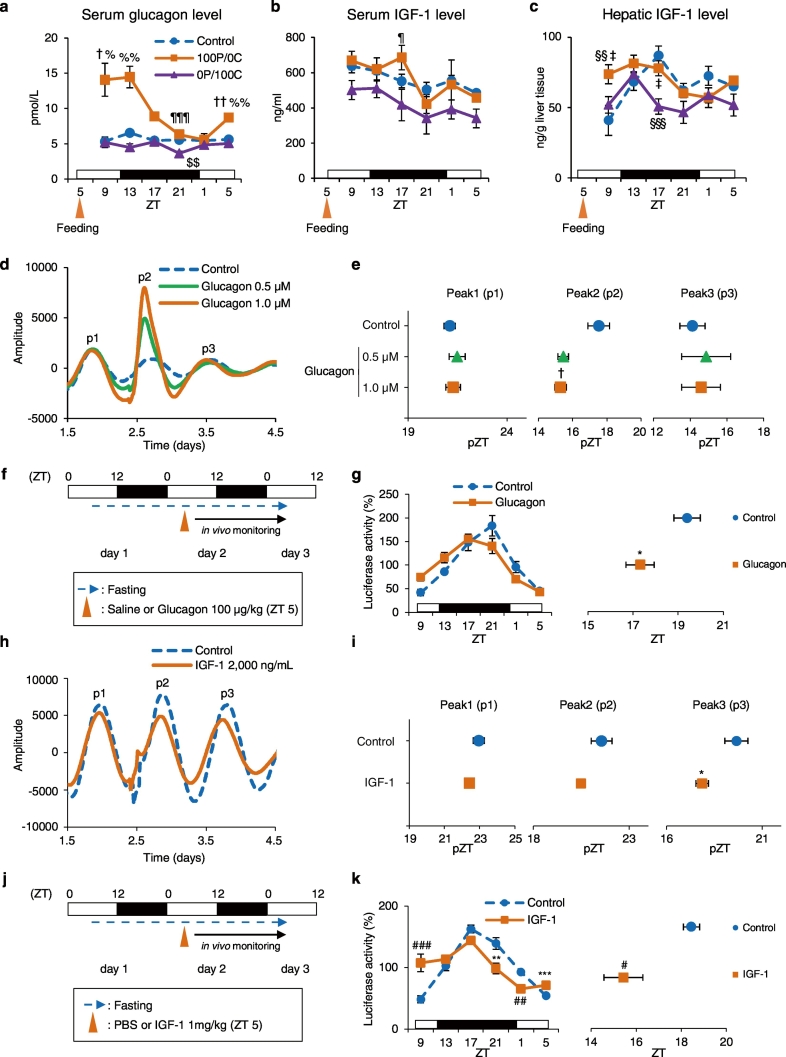
Fig. 2.
Protein-only diet increases glucagon and IGF-1 production, resulting in a phase advance both in vitro and in vivo. The serum or liver samples from the same individual as Fig. 1d: (a) serum glucagon levels, (b) serum IGF-1 levels, and (c) hepatic IGF-1 levels. Control, 100P/0C, and 0P/100C groups at each point: n = 5. (d) Representative PER2::LUC bioluminescence rhythms from liver slices explants of mice under free-feeding conditions. Liver slices were treated with sterile distilled water (Control), 0.5 μM, or 1.0 μM glucagon. Waveforms were smoothened and detrended. (e) Calculated peak phases from d; Control: n = 10; 0.5 μM: n = 9; 1.0 μM: n = 8. (f) Experimental schedule. Under free-feeding conditions, mice were treated with saline (Control group) or 100 μg/kg glucagon at ZT 5 after a 24-h starvation. The blue broken line indicates fasting; the black arrow indicates in vivo monitoring. (g) The left graph shows average relative waveforms of hepatic in vivo PER2::LUC bioluminescence in the Control and Glucagon groups. The right graph shows average peak phases of in vivo PER2::LUC rhythms; Control: n = 4; Glucagon: n = 5. (h) Representative PER2::LUC bioluminescence rhythms from liver slices explants of mice under free-feeding conditions. Liver slices were treated with PBS (Control) or 2000 ng/mL IGF-1. Waveforms were smoothened and detrended. (i) Calculated peak phases from h; Control: n = 6; IGF-1: n = 5. (j) Experimental schedule. Under free-feeding conditions, mice were treated with PBS (Control group) or 1 mg/kg IGF-1 at ZT 5 after a 24-hour starvation. The blue broken line indicates fasting, and the black arrow indicates in vivo monitoring. (k) The left graph shows average relative waveforms of hepatic in vivo PER2::LUC bioluminescence in the Control and IGF-1 groups. The right graph shows average peak phases of in vivo PER2::LUC rhythms; Control: n = 8; IGF-1: n = 10. Data are presented as the mean ± SEM. $$P < 0.01 (vs. Control), ¶P < 0.05, ¶¶¶P < 0.001 (vs. 0P/100C) by one-way ANOVA with Tukey post-hoc test; †P < 0.05, ††P < 0.01 (vs. Control), %P < 0.05, %%P < 0.01 (vs. 0P/100C) by Kruskal-Wallis test with Dunn post-hoc test; §§P < 0.01, §§§P < 0.001 (vs. Control), ‡P < 0.05 (vs. 0P/100C) by two-way ANOVA with Tukey post-hoc test; *P < 0.05, **P < 0.01, ***P < 0.001 (vs. Control) by the Student t-test; #P < 0.05, ##P < 0.01, ###P < 0.001 (vs. Control) by the Mann-Whitney test.