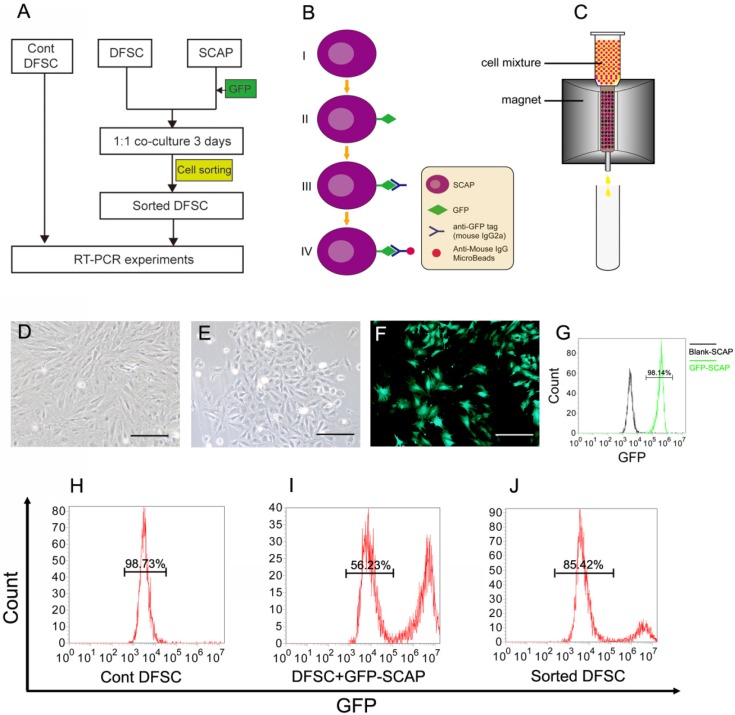
Figure 3.
DFSCs were co-cultured with GFP-labeled SCAPs and then sorted. (A) The co-culture experiment was designed to study the possible regulating role of SCAPs in differentiation of DFSCs. (B-C) The schematic figures showing how to label SCAPs to sort them out from the cell mixture. (B) Step I-II: SCAPs were transfected with recombinant retrovirus expressing GFP (RV-GFP). Step III: After the cell mixture (SCAPS:DFSCs = 1:1) was harvest, it was incubated with anti-GFP tag antibody (mouse IgG2a). Step IV: The mixture was incubated with anti-mouse IgG MicroBeads. (C) The cell mixture was loaded onto the column. The GFP+ SCAPs with MicroBeads (red) was attracted and retained inside the column in the magnetic field, while the unlabeled DFSCs (yellow) could pass through. After the magnetic field was removed, the retained SCAPs could be collected later. (D) DFSCs were isolated from DF tissue of DI3 at the stage of PN10 and cultured. (E) SCAPs were isolated from the apical papilla of DI3 at the stage of PN10 and cultured. (F) SCAPs were transfected with GFP retrovirus. (G) High efficiency of GFP transfection (98.14±3.3%) was examined. (H-J) After being sorted, the proportion of co-cultured DFSCs was elevated from 56.23±2.6% (before) to 85.42±3.6% (after). Scale bars represent 100μm.