ABSTRACT
Sapovirus (SaV), from the Caliciviridae family, is a genus of enteric viruses that cause acute gastroenteritis. SaV is shed at high concentrations with feces into wastewater, which is usually discharged into aquatic environments or reused for irrigation without efficient treatments. This study analyzed the incidence of human SaV in four wastewater treatment plants from Tunisia during a period of 13 months (December 2009 to December 2010). Detection and quantification were carried out using reverse transcription-quantitative PCR (RT-qPCR) methods, obtaining a prevalence of 39.9% (87/218). Sixty-one positive samples were detected in untreated water and 26 positive samples in processed water. The Dekhila plant presented the highest contamination levels, with a 63.0% prevalence. A dominance of genotype I.2 was observed on 15 of the 24 positive samples that were genetically characterized. By a Bayesian estimation algorithm, the SaV density in wastewater was estimated using left-censored data sets. The mean value of log SaV concentration in untreated wastewater ranged between 2.7 and 4.5 logs. A virus removal efficiency of 0.2 log was calculated for the Dekhila plant as the log ratio posterior distributions between untreated and treated wastewater. Multiple quantitative values obtained in this study must be available in quantitative microbial risk assessment in Tunisia as parameter values reflecting local conditions.
IMPORTANCE Human sapovirus (SaV) is becoming more prevalent worldwide and organisms in this genus are recognized as emerging pathogens associated with human gastroenteritis. The present study describes novel findings on the prevalence, seasonality, and genotype distribution of SaV in Tunisia and Northern Africa. In addition, a statistical approximation using Bayesian estimation of the posterior predictive distribution (“left-censored” data) was employed to solve methodological problems related with the limit of quantification of the quantitative PCR (qPCR). This approach would be helpful for the future development of quantitative microbial risk assessment procedures for wastewater.
KEYWORDS: Bayesian estimation, human sapovirus, RT-qPCR, Tunisia, genotyping, wastewater
INTRODUCTION
Wastewater reclamation systems constitute practical options to mitigate water scarcity in many countries for multiple purposes: irrigation, ground water recharge, recreational impoundment, and even as a drinking source. Enteric viruses represent a risk for such systems, as they are released to wastewater in enormous amounts with feces from symptomatic/asymptomatic individuals (1). The inappropriate use of reclaimed waters has caused outbreaks of viral infectious diseases worldwide, such as the norovirus (NoV) strawberry outbreak on eastern Germany in 2012 (2). It is important to efficiently reduce enteric viruses from wastewater prior to the usage of reclaimed wastewater for any purpose.
The reduction of enteric viruses in wastewater treatment plants (WWTPs), however, is mostly inefficient (3) and is relatively poor compared with that of fecal indicator microorganisms such as Escherichia coli (4). In several countries in Africa, including Tunisia, the quality of treated wastewater is still poor, even though advanced WWTPs have been installed (5). During the last decade, the insufficiently treated wastewater, contaminated with enteric viruses, has been used in Tunisia for agricultural irrigation and the refilling of aquifers to help palliate the lack of water resources (6); this combination of factors might become a threat for public health. Furthermore, the lack of a specific system in Tunisia for the surveillance of enteric viruses in wastewater is among the many factors that increase the potential risk for humans associated with viral contamination in wastewater (7, 8).
Sapovirus (SaV) is one of the enteric viruses that pose significant disease burden (9). Sapporo virus is the type species of the genus Sapovirus first detected in Japan (10) and classified within the Caliciviridae family (11). Outbreaks are less common than those caused by human NoV, and SaV affects people of all ages, although it is more prevalent in infants. The symptoms caused by both viruses, such as diarrhea, vomiting, abdominal pain, fever, chills, and headache, make them mostly indistinguishable. Low infectious doses, prolonged shedding, environmental stability, and high strain diversity increase the risk of infection (12), which occurs mainly by the fecal-oral route. The electron microscopy (EM) observation of the “Star of David” morphology on the surface of SaV enables the recognition of the viral particles, differentiating them from other enteric viruses (13). This technique and antigen detection methods such as enzyme-linked immunosorbent assay (ELISA) are not widely used nowadays because they have been shown to have low sensitivity compared with nucleic acid methods (14). The most utilized detection method for SaV is reverse transcription-quantitative PCR (RT-qPCR), which also allows the quantification of positive samples. However, information about SaV in the environment, such as the density in wastewater and removal efficiency in wastewater treatment processes, is scarce, and studies are limited to countries such as Japan, Italy, South Africa, Brazil, or the United States (15–19). The aim of the present study was to extend the limited knowledge about the prevalence of SaV in Tunisia, using for this purpose wastewater samples. In addition, quantitative and qualitative data may give important information about SaV circulation in this country. Finally, we aimed to analyze the genetic diversity by sequence characterization of the positive samples.
RESULTS
SaV detection in wastewater.
Extraction efficiencies of all samples were acceptable (>5%) as were RT-qPCR efficiencies (>25%) according to ISO technical specification (ISO/TS) 15216-1:2013. All four WWTPs showed contamination with human SaV, which was detected in 87 out of 218 (39.9%) samples (Table 1). When the positive rates from raw and treated wastewater were compared, a higher percentage of viral contamination was observed in influent points, which showed 61 positive samples out of 109 samples (56.0%), while effluent points showed only 26 positives (23.9%).
TABLE 1.
Detection and quantification (GC/liter wastewater) of sapovirus (SaV) in influent and effluent wastewater samples
| WWTP | No. analyzed | Detected |
Density (GC/liter wastewater) |
||||
|---|---|---|---|---|---|---|---|
| No. positive | % positive | Total no. (%) positive | Avg in positive samples | Range |
|||
| Minimum | Maximum | ||||||
| Sidi-Bouzid | 21 (38.9) | 2.7 × 104 | 4.5 × 108 | ||||
| Influent | 27 | 15 | 55.5 | 4.5 × 106 | 2.7 × 104 | 4.5 × 108 | |
| Effluent | 27 | 6 | 22.2 | 1.2 × 107 | 5.8 × 105 | 1.4 × 108 | |
| Sbeitla | 19 (35.2) | 5.8 × 103 | 5.3 × 108 | ||||
| Influent | 27 | 18 | 66.6 | 3.0 × 105 | 5.8 × 103 | 5.3 × 108 | |
| Effluent | 27 | 1 | 3.7 | 4.1 × 107 | 4.1 × 107 | 4.1 × 107 | |
| M'saken | 13 (23.2) | 3.0 × 104 | 6.9 × 106 | ||||
| Influent | 28 | 11 | 39.3 | 1.9 × 105 | 3.0 × 104 | 6.9 × 106 | |
| Effluent | 28 | 2 | 7.1 | NQPa | NQP | NQP | |
| Dekhila | 34 (63.0) | 4.3 × 103 | 1.9 × 108 | ||||
| Influent | 27 | 17 | 63.0 | 1.1 × 106 | 4.3 × 103 | 1.9 × 108 | |
| Effluent | 27 | 17 | 63.0 | 2.4 × 105 | 5.2 × 103 | 1.5 × 108 | |
| Total | 87 (39.9) | 4.3 × 103 | 5.3 × 108 | ||||
| Influent | 109 | 61 | 56.0 | 7.8 × 105 | 4.3 × 103 | 5.3 × 108 | |
| Effluent | 109 | 26 | 23.9 | 9.3 × 105 | 5.2 × 103 | 1.5 × 108 | |
NQP, nonquantifiable positives.
Dekhila WWTP was, by far, the most contaminated plant, with a SaV prevalence of 63.0%, while M'saken WWTP was the least contaminated (23.2%). Both plants are located near the Mediterranean coast, whereas the other two WWTPs (Sidi-Bouzid and Sbeitla) are located in inner regions of the country and showed rates of 38.9% and 35.2%, respectively. Positive sample detection decreased after primary and secondary treatments in all plants except in the Dekhila WWTP, where raw and treated water both showed 17 contaminated samples.
Quantification and log reduction.
Human SaV geometric mean quantification for all WWTP points was 7.0 × 105 genomic copies (GC)/liter wastewater, with a minimum of 4.3 × 103 GC/liter wastewater detected in Dekhila WWTP (for all WWTPs, only samples above the quantification limit were used) and a maximum of 5.3 × 108 GC/liter wastewater detected in the Sbeitla WWTP. Sidi-Bouzid presented the highest average quantification with 5.7 × 106 GC/liter wastewater.
The posterior predictive distributions of all the influents in the WWTPs are shown in Fig. 1A. Prior to the parameter estimation, data sets were tested using the Bayesian information criterion (BIC), which clarified that the better fitting distribution was achieved by log-normal rather than normal and gamma distributions (data not shown). Logarithmic mean values were estimated to be around 4.0 to 4.5 for all WWTP influents except that from M'saken, which was 2.7. The log mean value in the posterior predictive distribution of the Dekhila effluent (Fig. 1B) was 3.7. Logarithmic standard deviations (SD) from all influents and the Dekhila effluent ranged between 2.5 and 4.3.
FIG 1.
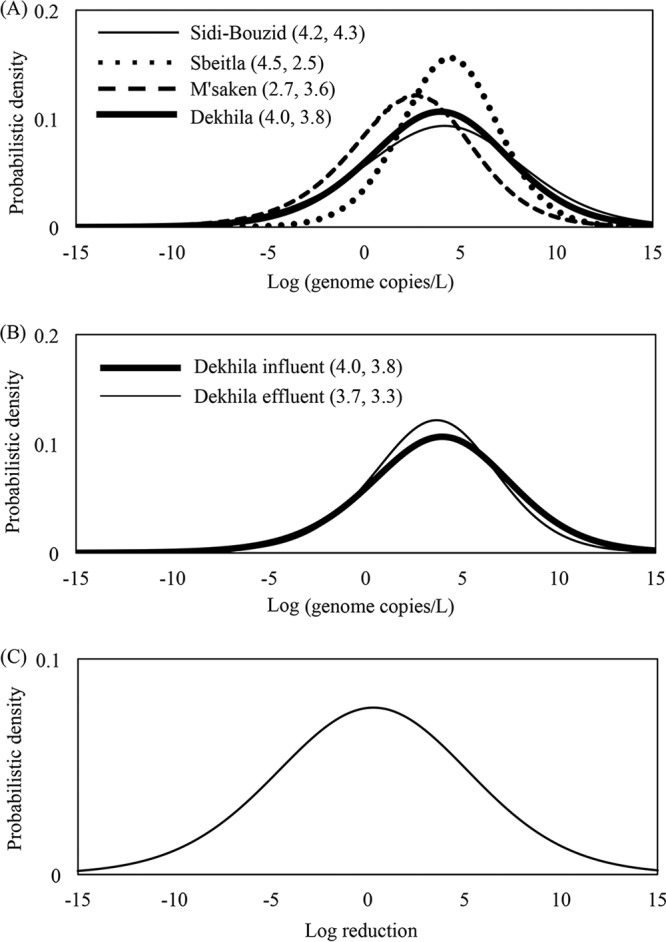
Posterior predictive distributions of sapovirus (SaV) density in all the WWTP influents (A) and in influent and effluent from the Dekhila plant (B) (numbers in parentheses indicate logarithmic mean values and standard deviations). (C) Log-ratio virus removal in the Dekhila WWTP.
Considering all plants together, no reductions of SaV load were observed from raw wastewater compared to treated wastewater when SaV was detected. When observing each plant separately, only in Dekhila and in M'saken were reductions of the average viral concentration from influents observed, as the Sidi-Bouzid and Sbeitla plants presented higher concentrations in their outflows (Table 1). The virus log reduction was estimated by the Bayesian model only for the Dekhila plant because eight or more positive samples are needed to achieve an estimate with good accuracy, and due to the detection results obtained, not all the effluents were suitable to be used in the Bayesian estimation. Log reduction calculated using a log ratio of the posterior distributions of Dekhila influent and effluent is shown on Fig. 1C, where the log mean of the viral removal was 0.2.
Seasonality.
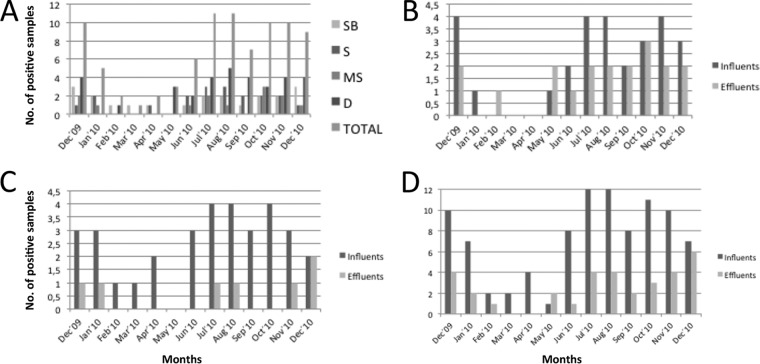
The prevalence data obtained were analyzed in order to study any possible seasonality during the 13 months of the study (Fig. 2A). July and August 2010 were the two months with the most contamination, with 11 positive samples in each. On the other hand, March 2010 presented the lowest rate, with only 1 positive sample, followed by February and April, with 2 each. The general trend observed is a decrease in contamination after January 2010 and a significant overall increase after May 2010. When looking at the entry and exit positive samples from both coastal and interior WWTPs, we observed the same trend mentioned above. In general, we observed the same tendency, i.e., an increase in positive samples, on entries and on exits, both overall and by region (Fig. 2B to D).
FIG 2.
Seasonality of SaV represented by the number of positive samples among the months of study at each WWTP (A), at coastal WWTPs (B) and at interior WWTPs (C) for both influents and effluents and for influents and effluents shown separately (D). SB, Sidi-Bouzid; S, Sbeitla; MS, M'saken; D, Dekhila.
Genetic characterization.
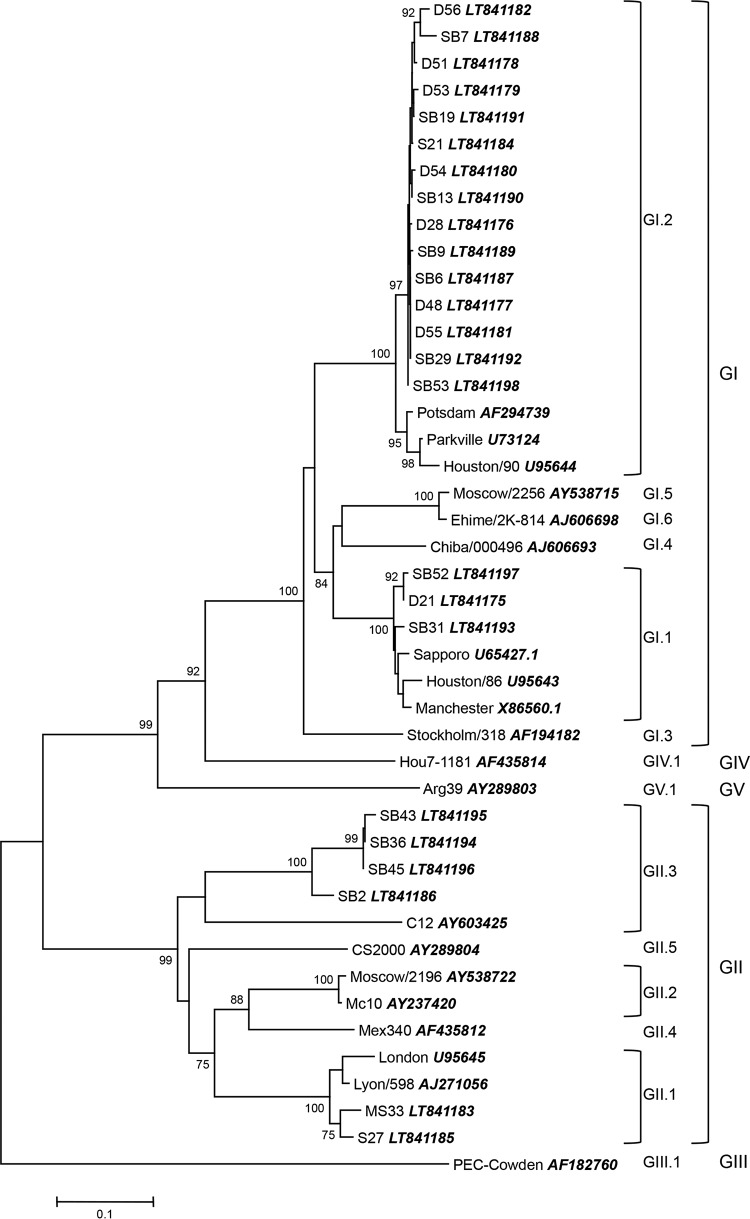
Out of the 87 positive samples, only 24 were successfully genotyped. Phylogenetic analysis of the sequences obtained were conducted and a neighbor-joining tree was constructed (Fig. 3). Genogroup I (75.0%) was the predominant type in these samples, specifically genotype GI.2 (62.5%), with 15 samples. Genogroup II was also detected in six samples, mostly genotype GII.3 (16.7%). It is worth noting that practically the same distribution of genotypes was observed between the coastal region and the interior region: 1 sample GI.1, 7 or 8 samples GI.2, and 1 or 2 samples GII.1; genotype GII.3 was the exception because it was detected only in the inner WWTPs.
FIG 3.
Neighbor-joining phylogenetic tree constructed using MEGA 6 based on the partial capsid gene sequences of SaV detected samples. Bootstrap values are shown at each node as percentages of 1,000 replicates (greater than 70%). GenBank accession numbers of the reference strains are detailed in the tree.
DISCUSSION
The present study shows the prevalence and epidemiology of human SaV in Tunisian territory. Four WWTPs from different regions of the country, some of them with tourism importance, were analyzed during 13 months. The primary aim of the study was helping to evaluate the risk that this enteric virus may present in the reutilization of treated wastewater and the implications for its transmission. Characterization of this enteric virus in wastewater provides information on its circulation among both symptomatic and asymptomatic populations, which is useful because no routine surveillance is taking place.
Influent samples showed a 56.0% positive percentage, which is significantly lower than that in other studies carried out in Japan and the United States, where SaV was detected in almost all the influent samples (15, 19, 20), but higher than that in similar surveillances in the Mediterranean region (16) or in South America (18). Effluents showed a detection rate of 23.9%, which is low compared to rates in Japanese (conventional activated sludge) and U.S. (conventional activated sludge and biological trickling filter process) studies (15, 19, 20), but still higher than the rate in Brazilian studies, where no SaV was detected in final effluents (conventional activated sludge) (18). General detection rates in Tunisia are lower than those in South Africa (17), higher than those in Italy (16), and similar to those in Brazil (18).
WWTPs in the coastal region presented the highest (Dekhila) and lowest (M′saken) detection rates, 63.0 and 23.2%, respectively (activated sludge). The viral rate detected in the central Tunisian WWTPs was 38.9% for Sidi-Bouzid (optional aerated lagoon) and 35.2% for Sbeitla (activated sludge). These results are partially in line with those found in previous studies (5, 21), where hepatitis A virus (HAV) detection was in general higher in inland regions of the country.
The results obtained are not in accordance with the general belief that the peak of detection of SaV occurs in cold and rainy months (15, 18, 22) and also not in accordance with the seasonal prevalence observed in Tunisia for NoV (23). Nevertheless, the predominant trend on our study was a higher number of SaV-contaminated samples detected during the summer season, both in coastal and in inland WWTPs. These results can be explained by the increase in tourist activities from both domestic and the European Union tourists (5, 21).
Studies conducted in other countries about quantification of SaV are scarce. In those, the maximum values of load concentrations were 104 to 106 GC/liter wastewater (15, 18–20), which are not in agreement with our results. All Tunisian WWTPs presented maximum quantifications between 106 and, mostly, 108 GC/liter wastewater. The mean values of both influents and effluents was close to 106 GC/liter wastewater, demonstrating that the difference in the concentration of quantified virus from untreated and treated wastewater was not appreciable.
SaV loads detected at exit points demonstrate the difficulty of some of the WWTPs in achieving a proper removal of enteric viruses by the usual wastewater biological treatments. Both Sbeitla (coastal) and M'saken (interior) (both activated sludge) were the units with the highest removal efficiency, as they were able to reduce by 94.4% and 81.8% the SaV presence, respectively. Sidi-Bouzid (optional aerated lagoon) had some difficulties during autumn and winter, as the detection of SaV in its effluent waters persisted. However, the real issue was observed in the Dekhila WWTP (activated sludge), where the general removal percentage not only did not rise above 15% but also was null during many parts of the study.
Most of the WWTPs reported in previous studies use conventional activated sludge (18–20), some of them followed by chlorination (15), and their results are in line with those obtained for Sbeitla and M′saken. The optional aerated lagoon process adopted at Sidi-Bouzid did not seem to yield better results than the activated sludge process, which is widely used by cities and communities where large volumes of wastewater need to be treated in a cost-saving manner.
In contrast to our study, a higher genetic diversity of human SaV was detected in the studies conducted in Japan, South Africa, and Spain (17, 20, 24), while in Italy and Brazil, genotypes GI.1 and GI.2, the predominant genotypes worldwide, were chiefly detected (16, 18). Only four genotypes of SaV were characterized in this study in Tunisia. It is important to mention that the approach utilized here was direct sequencing, and that sequencing after cloning could have shown a wider variety of genotypes. Wastewater was expected to display a broader range of types shed by human population, particularly based on our previous experience (25, 26) and considering that various WWTPs were examined. The detection of GI.1 and GI.2 shows that they continue to be the most prevalent worldwide and are mainly associated with outbreaks and sporadic cases (27–30).
Quantification data for viruses in wastewater commonly include a significant number of nondetects (31). Depending on environmental and physical factors, such as the lack of homogeneity of viral particles in water bodies, formation of aggregates with organic matter, or binding to suspended solids (32), virus density in wastewaters may fall below the quantification limit (nondetect) of the analytical methods employed. On the other hand, spatial and temporal variation in virus density in water samples is inevitable, so results with a high number of nondetects (left-censored data sets) are normally obtained. A Bayesian modeling approach has been adapted for left-censored data of enteric virus in wastewater samples, offering inference results in the form of probabilistic distributions and information about how a certain value is well estimated (33). It also can be used for expressing the virus removal efficiency of a treatment unit by dividing one posterior predictive distribution (i.e., untreated water of the influent) by the other (i.e., treated water of the effluent), obtaining a log ratio posterior distribution to express the variation (22). That is why we treated our data sets, including nondetect (left-censored) data, analyzing them with a Bayesian estimation of the posterior predictive distribution, using this method on a real surveillance for SaV. Based on these results, it should be noted how the log mean value of all influents from all WWTPs seems to be reduced by about 1.5 to 2.0 log units, based on the statistical approach prediction. That is due to the estimation of those nondetected samples under the quantification limit.
The ratio of influents to effluents in the Dekhila WWTP showed how this unit is totally inefficient for both the decrease of prevalence and the reduction of its load concentration, with a reduction of only 0.2 log mean value. Although it was reported in a previous study (34) that SaV in wastewater was well removed by membrane bioreactor, this study showed that the reduction efficiency of SaV may largely depend on treatment process type.
The concentration of SaV in WWTP influents presents a positive correlation with infected patients according to Haramoto (15), suggesting that the influents would be an appropriate target to understand the exact occurrence of SaV in the area. In that study some samples were also detected when no isolation cases were detected, suggesting viral loads from gastroenteritis were not being reported or were coming from asymptomatic people. Considering all of this, WWTPs might be considered one of the most effective observation points for controlling the presence of viruses circulating in human society, as they are less biased than clinical observation points.
Tunisia has developed its WWTPs only to secondary-level treatments. Our study showed that SaV is widely circulating in the country, in accordance with previous results for other enteric viruses, such as HAV (5, 21). Tunisia does not have a gastroenteritis outbreak reporting system or routine surveillance, and it has been suggested that socioeconomic status and hygiene level of the population are key points in the epidemiologic pattern of enteric viruses, due to the seroprevalence observed in rural areas (35, 36). Other developing countries in the region such Morocco, Algeria, or Egypt employ the same treatment processes (37). An observed trend in all of these countries is the increase in urban populations, which leads to the failure of the WWTPs to process such volumes of wastewater. Nowadays, the Tunisian government is putting efforts into the upgrade to tertiary-level treatments (38), but meanwhile treated wastewater is discharged into the sea or used for agriculture or irrigation. This surveillance may help generate data on SaV circulation in the population.
Human SaV is becoming more prevalent worldwide and viruses of this genus are recognized as emerging pathogens associated with human gastroenteritis (20). The present study describes novel findings on the prevalence, seasonality, and genotype distribution of SaV in Tunisia and Northern Africa. Analysis of wastewaters from different communities around the country reveals a frequent occurrence of SaV that indicates a more extensive circulation than previously suggested (39). Treatment processes used in Tunisia nowadays have shown, in some cases, inefficient removal of SaV from wastewater that may constitute a source of environmental contamination with potentially infectious viruses. We also provide comprehensive data of a statistical approximation using Bayesian modeling, which is needed to solve methodological problems related to the limit of quantification of the qPCR and the obtaining of left-censored data. This study should be taken in consideration for the future development of official controls using quantitative microbial risk assessment, especially to improve the reuse of treated wastewater.
MATERIALS AND METHODS
Sampling, viral concentration, and RNA extraction.
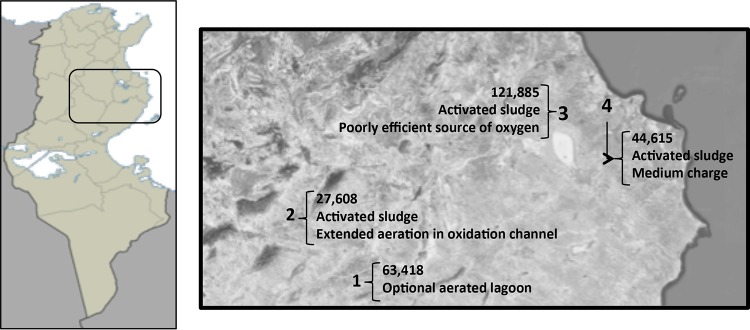
For this study, four Tunisian WWTPs were selected, two of them in the center of the country (Sidi-Bouzid and Sbeitla) in a non-touristic area with semiarid climate conditions and the other two on the east-central coast (M'saken and Dekhila), near the tourist cities of Monastir and Sousse, with Mediterranean weather (Fig. 4). All WWTPs follow the requirements of the Tunisian law on environmental impact assessment and regulations on the quality of discharges and reuse of treated water. Physicochemical indicators to determine the quality parameters as well as the WWTP processes and characteristics of each area according to the National Office of Sanitation (ONAS) of Tunisia were described in more depth elsewhere (5, 21).
FIG 4.
Location of the WWTPs in Tunisia. 1, Sidi-Bouzid; 2, Sbeitla; 3, M'saken; 4, Dekhila. City population and WWTP treatment shown on the map. The template map used for the left panel is from MapaMundial.co (http://mapamundial.co/a/mapadeTunez#mapa). Aerial view map obtained from Google Maps (Imagery@2016 Landsat, Data SIO, NOAA, U.S. Navy, NGA, GEBCO, map data @Google) under the Fair Use terms.
A total of 218 samples (2 liters of wastewater) from influent and effluent points of the WWTPs were obtained every 15 days during 13 months (December 2009 to December 2010) (5, 21). Original viral concentrates, obtained by the adsorption-elution method recommended by the U.S. Environmental Protection Agency (1992) (40) and kept at −80°C, were employed. Briefly, aluminum chloride (AlCl3) was added to 100 ml of wastewater sample and adjusted to a pH of 3.5 with 5 N HCl. Viruses were then eluted by beef extract, pH 9, and concentrated by precipitation using polyethylene glycol (PEG) 6000. The pellets were resuspended in 3 ml of phosphate-buffered saline (PBS) and used immediately or stored at −80°C until use (21). Viral RNA was extracted from 150 μl of the concentrates using the NucleoSpin RNA virus kit (Macherey-Nagel, Germany) and eluted in a final volume of 50 μl of H2O RNase free. Known amounts of mengovirus clone (vMC0) (10 μl of vMC0 stock, 103 vMC0 PFU) were previously spiked to each sample as an independent nucleic acid extraction efficiency control (41).
RT-qPCR.
Detection and quantification, determination of efficiencies, and limit of detection were settled following the standards outlined in ISO/TS 15216-1:2013 (41), with minor modifications as explained in each section. The RT-qPCR was carried out using SaV124F (0.4 μM) and SaV1245R (0.4 μM) primers and SaV124TP probe (0.2 μM) (Table 2), which target the polymerase-capsid junction in open reading frame (ORF)-1, as described by Oka et al. (42). This would amplify human genogroups I, II, and IV using a Platinum Quantitative RT-PCR Thermoscript One-step System kit. Each sample was tested in duplicates. Reaction was performed in a final volume of 25 μl, and cycling conditions for the reverse transcription were 20 min at 50°C and 15 min at 95°C, followed by 45 cycles of qPCR: 15 s denaturalization at 95°C, 1 min of annealing at 50°C, and elongation. The presence of inhibitors within the RT-qPCR was analyzed with the inclusion of plasmid external controls (obtained as explained below) in separate wells of the plate, as described elsewhere (41). Any sample with less than 5% of extraction efficiency was tested again, as well as any with less than 25% of PCR efficiency. Maximum cycle threshold (Ct) for a positive sample to be quantified was 39, while values between 39 and 41 were outside the quantification range but considered positive; all samples above Ct 41 were considered negatives.
TABLE 2.
Primers and probes employed for RT-qPCR and RT-nested PCR
| PCR type | Primer/probe | Sequence (5′–3′)a | Polarityb | Location (nt) | Reference |
|---|---|---|---|---|---|
| RT-qPCR | SaV124F | GAYCASGCTCTCGCYACCTAC | + | 5078–5098c | 42 |
| SaV1245R | CCCTCCATYTCAAACACTA | − | 5181–5163d | 42 | |
| SaV124TP | FAM-CCCCTATRAACCA-MGB-NFQ | − | 5112–5135d | 42 | |
| Nested RT-PCR | SV-R13 | GGTGANAYNCCATTKTCCAT | − | 5876–5861e | 47 |
| SV-R14 | GGTGAGMMYCCATTCTCCAT | − | 5876–5861e | 47 | |
| SaV1F | TTGGCCCTCGCCACCTAC | + | 700–717f | 42 | |
| SaV5F | TTTGAACAAGCTGTGGCATGCTAC | + | 5112–5135d | 42 | |
| SaV124F | GAYCASGCTCTCGCYACCTAC | + | 5078–5098c | 42 | |
| SV-R2 | GWGGGRTCAACMCCWGGTGG | − | 5591–5572e | 47 | |
| 1245Rfwd | TAGTGTTTGARATGGAGGG | + | 5163–5181c | 43 |
Y, C or T; S, G or C; N, A, C, G, or T; K, G or T; M, A or C; W, A or T; R, A or G.
+, sense; −, antisense.
Position according to Mc10 virus (AY237420).
Position according to NK24 virus (AY646856).
Position according to Manchester virus (X86560).
Position according to Parkville virus (U73124).
The limit of detection (1.6 × 103 RNA copies per liter of wastewater) and standard curves for the qPCR were performed cloning a 112-bp fragment into pGEM-T Easy plasmid and transformed into competent Escherichia coli DH5α cells. The fragment corresponded to the polymerase-capsid junction of SaV (nucleotide positions 5073 to 5184) of GI Sapporo strain full-length genome (GenBank accession number HM002617) and the plasmid were quantified using spectrophotometry (A260). No correction or back calculation was calculated on the quantification results using extraction or PCR efficiencies, as addressed in the ISO procedures (41).
Genetic characterization and phylogenetic analysis.
The SaV RNAs detected were subjected to genotyping by reverse transcription (RT)-nested PCR (25, 43). First, an RT was carried out using 5 μl of viral RNA and 20 μl of mixture (RevertAid reverse transcriptase kit) containing primers SV-R13 and SV-R14 (1 μM). Reaction conditions were 42°C for 60 min.
Outer PCR used forward primers SaV124F, SaV1F, and SaV5F; reverse primers SV-R13 and SV-R14 (0.5 μM each); and 5 μl of viral cDNA obtained on the RT. Conditions were 2 min at 94°C, 40 cycles: 30 s at 94°C, 30 s at 50°C, 2 min at 72°C, and finally 10 min at 72°C. Inner PCR consisted of 45 cycles under similar conditions using primers SV-R2 and 1245Rfwd (0.5 μM each), adding 5 μl of product from the previous outer PCR. Both PCRs were performed using a Fermentas DreamTaq DNA polymerase kit. All primers used for the genetic characterization are shown in Table 2.
Nested PCR products of approximately 420 bp were sequenced at STAB Vida Laboratory (Portugal). Data obtained were processed using a Lasergene 7 software package, aligned using a MEGA version 6 software package (44), and phylogenetic dendrograms were built by the neighbor-joining and maximum-likelihood methods with a bootstrap analysis of 1,000 replicates. Reference strain sequences were downloaded from GenBank.
Bayesian estimation of the concentration distribution and log reduction.
A Bayesian estimation algorithm was applied to estimate posterior predictive distributions of SaV density in influent and effluent using left-censored data sets, which included nondetected samples (33). The applicability of the Bayesian model to the data sets obtained in this study is subject to how well data sets fit to probabilistic distribution, which was tested using the Bayesian information criterion (BIC) (45). Better fitting with the virus concentration was determined by the lowest BIC values, which were calculated using R code (46). The estimation accuracy is strongly affected by the number of those detects: data with eight or more are required to estimate accurately the posterior predictive distributions (22). A log ratio posterior distribution, regarded as virus removal efficiency, was also calculated by dividing two posterior predictive distributions of virus density in influent and effluent samples (22, 33). The software implementing the algorithm developed for inferring posterior predictive distribution of virus concentration and virus removal efficiency is available upon request.
Accession number(s).
Sequences of SaV strains detected in the present study are available at GenBank under accession numbers LT841175 to LT841198.
ACKNOWLEDGMENTS
We appreciate Toshihiro Ito, Kyoto University, for his technical help.
This study was supported, in part, by grant 2014-PG110 from the Xunta de Galicia (Spain).
The authors declare no competing financial interest.
REFERENCES
- 1.Ozawa K, Oka T, Takeda N, Hansman GS. 2007. Norovirus infections in symptomatic and asymptomatic food handlers in Japan. J Clin Microbiol 45(12):3996–4005. doi: 10.1128/JCM.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mäde D, Trübner K, Neubert E, Höhne M, Johne R. 2013. Detection and typing of norovirus from frozen strawberries involved in a large-scale gastroenteritis outbreak in Germany. Food Environ Virol 5:162–168. doi: 10.1007/s12560-013-9118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Silva AK, Le Saux JC, Parnaudeau S, Pommepuy M, Eimelech M, Le Guyader FS. 2007. Evaluation of removal of norovirus during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of genogroups I and II. Appl Environ Microbiol 73(24):7891–7897. doi: 10.1128/AEM.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottoson J, Hansen A, Björlenius B, Norder H, Stenström TA. 2006. Removal of viruses, parasitic protozoa and microbial indicators in conventional and membrane processes in a wastewater pilot plant. Water Res 40:1449–1457. doi: 10.1016/j.watres.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Ouardani I, Turki S, Aouni M, Romalde JL. 2016. Detection and molecular characterization of hepatitis A virus from Tunisian wastewater treatment plants with different secondary treatments. Appl Environ Microbiol 82:3834–3845. doi: 10.1128/AEM.00619-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Ayni F, Manoli E, Cherif S, Jrad A, Assimacopoulus D, Trabelsi-Ayadi M. 2013. Deterioration of a Tunisian coastal aquifer due to agricultural activities and possible approaches for better water management. Water Environ J 27:348–361. [Google Scholar]
- 7.Sdiri-Loulizi K, Hassine M, Aouni Z, Gharbi-Khelifi H, Chouchane S, Sakly N, Neji-Guédiche M, Pothier P, Aouni M, Ambert-Balay K. 2010. Detection and molecular characterization of enteric viruses in environmental samples in Monastir, Tunisia between January 2003 and April 2007. J Appl Microbiol 109:1093–1104. doi: 10.1111/j.1365-2672.2010.04772.x. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Lázaro D, Cook N, Ruggeri FM, Sellwood J, Nasser A, Nascimento MS, D′Agostino M, Santos R, Saiz JC, Rzeżutka A, Bosch A, Gironés R, Carducci A, Muscillo M, Kovač K, Diez-Valcarce M, Vantarakis A, von Bonsdorff CH, de Roda Husman AM, Hernández M, van der Poel WH. 2012. Virus hazards from food, water and other contaminated environments. FEMS Microbiol Rev 36:786–814. doi: 10.1111/j.1574-6976.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grytdal SP, DeBess E, Lee LE, Blythe D, Ryan P, Biggs C, Cameron M, Schmidt M, Parashar UD, Hall AJ. 2016. Incidence of norovirus and other viral pathogens that cause acute gastroenteritis (AGE) among Kaiser Permanente member populations in the United States, 2012-2013. PLoS One 11(4):e0148395. doi: 10.1371/journal.pone.0148395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba S, Sakuma Y, Kogasaka R, Akihara M, Horino K, Nakao T, Fukui S. 1979. An outbreak of gastroenteritis associated with calicivirus in an infant home. J Med Virol 4:249–254. doi: 10.1002/jmv.1890040402. [DOI] [PubMed] [Google Scholar]
- 11.Mayo MA. 2002. A summary of taxonomic changes recently approved by ICTV. Arch Virol 147:1655–1663. doi: 10.1007/s007050200039. [DOI] [PubMed] [Google Scholar]
- 12.Costantini V, Loisy F, Joens L, Le Guyader FS, Saif LJ. 2006. Human and animal enteric caliciviruses in oysters from different coastal regions of the United States. Appl Environ Microbiol 72(3):1800–1809. doi: 10.1128/AEM.72.3.1800-1809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madeley CR. 1979. Comparison of the features of astrovirus and caliciviruses seen in samples of feces by electron microscopy. J Infect Dis 139:519–523. doi: 10.1093/infdis/139.5.519. [DOI] [PubMed] [Google Scholar]
- 14.Hansman GS, Oka T, Katayama K, Takeda N. 2007. Human sapoviruses: genetic diversity, recombination, and classification. Rev Med Virol 17:133–141. doi: 10.1002/rmv.533. [DOI] [PubMed] [Google Scholar]
- 15.Haramoto E, Katayama H, Phanuwan C, Ohgaki S. 2008. Quantitative detection of sapoviruses in wastewater and river water in Japan. Lett Appl Microbiol 46:408–413. doi: 10.1111/j.1472-765X.2008.02330.x. [DOI] [PubMed] [Google Scholar]
- 16.Di Bartolo I, Ponterio E, Battistone A, Bonomo P, Cicala A, Mercurio P, Triassi M, Pennino F, Fiore L, Ruggeri FM. 2013. Identification and genotyping of human sapoviruses collected from sewage water in Naples and Palermo, Italy, in 2011. Food Environ Virol 5:236–240. doi: 10.1007/s12560-013-9124-2. [DOI] [PubMed] [Google Scholar]
- 17.Murray TY, Mans J, Taylor MB. 2013. Human calicivirus diversity in wastewater in South Africa. J Appl Microbiol 114:1843–1853. doi: 10.1111/jam.12167. [DOI] [PubMed] [Google Scholar]
- 18.Fioretti JM, Rocha MS, Fumian TM, Ginuino A, da Silva TP, de Assis MR, Rodrigues JDS, Carvalho-Costa FA, Miagostovich MP. 2016. Occurrence of human sapovirus in wastewater and stool samples in Rio de Janeiro, Brazil. J Appl Microbiol 121:855–862. doi: 10.1111/jam.13205. [DOI] [PubMed] [Google Scholar]
- 19.Kitajima M, Iker BC, Pepper IL, Gerba CP. 2014. Relative abundance and treatment reduction of viruses during wastewater treatment processes–identification of potential viral indicators. Sci Total Environ 488-489: 290–296. doi: 10.1016/j.scitotenv.2014.04.087. [DOI] [PubMed] [Google Scholar]
- 20.Kitajima M, Haramoto E, Phanuwan C, Katayama H. 2011. Genotype distribution of human sapoviruses in wastewater in Japan. Appl Environ Microbiol 77:4226–4229. doi: 10.1128/AEM.00088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouardani I, Manso CF, Aouni M, Romalde JL. 2015. Efficiency of hepatitis A virus removal in six sewage treatment plants from central Tunisia. Appl Microbiol Biotechnol 99:10759–10769. doi: 10.1007/s00253-015-6902-9. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Kato T, Takagishi K, Okabe S, Sano D. 2015. Bayesian modeling of virus removal efficiency in wastewater treatment processes. Water Sci Technol 72:1789–1795. doi: 10.2166/wst.2015.402. [DOI] [PubMed] [Google Scholar]
- 23.Hassine-Zaafrane M, Sdiri-Loulizi K, Kaplon J, Salem IB, Pothier P, Aouni M, Ambert-Balay K. 2013. Prevalence and genetic diversity of norovirus infection in Tunisian children (2007-2010). J Med Virol 85:1100–1110. doi: 10.1002/jmv.23552. [DOI] [PubMed] [Google Scholar]
- 24.Sano D, Pérez-Sautu U, Guix S, Pintó RM, Miura T, Okabe S, Bosch A. 2011. Quantification and genotyping of human sapoviruses in the Llobregat river catchment, Spain. Appl Environ Microbiol 77:1111–1114. doi: 10.1128/AEM.01721-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varela MF, Polo D, Romalde JL. 2016. Prevalence and genetic diversity of human sapovirus in shellfish from commercial production areas in Galicia, Spain. Appl Environ Microbiol 82:1167–1172. doi: 10.1128/AEM.02578-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varela MF, Hooper AS, Rivadulla E, Romalde JL. 2016. Human sapovirus in mussels from Ría do Burgo, A Coruña (Spain). Food Environ Virol 8:187–193. doi: 10.1007/s12560-016-9242-8. [DOI] [PubMed] [Google Scholar]
- 27.Medici MC, Tummolo F, Albonetti V, Abelli LA, Chezzi C, Calderaro A. 2012. Molecular detection and epidemiology of astrovirus, bocavirus, and sapovirus in Italian children admitted to hospital with acute gastroenteritis, 2008-2009. J Med Virol 84:643–650. doi: 10.1002/jmv.23231. [DOI] [PubMed] [Google Scholar]
- 28.Bucardo F, Reyes Y, Svensson L, Nordgren J. 2014. Predominance of norovirus and sapovirus in Nicaragua after implementation of universal rotavirus vaccination. PLoS One 9(5):e98201. doi: 10.1371/journal.pone.0098201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iritani N, Yamamoto SP, Abe N, Kubo H, Oka T, Kaida A. 2016. Epidemics of GI.2 sapovirus in gastroenteritis outbreaks during 2012-2013 in Osaka City, Japan. J Med Virol 88:1187–1193. doi: 10.1002/jmv.24451. [DOI] [PubMed] [Google Scholar]
- 30.Yoneda M, Nakano M, Sugimoto D, Inada M, Fujitani M, Kitahori Y. 2017. Epidemiological characteristics of sapovirus and human astrovirus detected among children in the Nara Prefacture, Japan, during 2009/2010-2014/2015 seasons. Jpn Infect Dis 70:87–91. doi: 10.7883/yoken.JJID.2015.529. [DOI] [PubMed] [Google Scholar]
- 31.Sano D, Amarasiri M, Hata A, Watanabe T, Katayama H. 2016. Risk management of viral infectious diseases in wastewater reclamation and reuse: review. Environ Int 91:220–229. doi: 10.1016/j.envint.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva AK, Le Guyader FS, Le Saux J-C, Pommepuy M, Montgomery MA, Elimelech M. 2008. Norovirus removal and particle association in a waste stabilization pond. Environ Sci Technol 42:9151–9157. doi: 10.1021/es802787v. [DOI] [PubMed] [Google Scholar]
- 33.Kato T, Miura T, Okabe S, Sano D. 2013. Bayesian modeling of enteric virus density in wastewater using left-censored data. Food Environ Virol 5:185–193. doi: 10.1007/s12560-013-9125-1. [DOI] [PubMed] [Google Scholar]
- 34.Miura T, Okabe S, Nakahara Y, Sano D. 2015. Removal properties of human enteric viruses in a pilot-scale membrane bioreactor (MBR) process. Water Res 75:282–291. doi: 10.1016/j.watres.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 35.Letaief A, Kaabia N, Gaha R, Bousaadia A, Lazrag F, Trabelsi H, Ghannem H, Letaief J. 2005. Age-specific seroprevalence of hepatitis A among school children in central Tunisia. Am J Trop Med Hyg 73:40–43. [PubMed] [Google Scholar]
- 36.Rezig D, Ouneissa R, Mhiri L, Mejri S, Haddad-Boubaker S, Ben Alaya N, Triki H. 2008. Seroprevalence of hepatitis A and E infectious in Tunisia. Pathol Biol 56:148–153. doi: 10.1016/j.patbio.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Qadir M, Bahri A, Sato T, Al-Karadsheh E. 2010. Wastewater production, treatment, and irrigation in Middle East and North Africa. Irrig Drainage Syst 24:37–51. doi: 10.1007/s10795-009-9081-y. [DOI] [Google Scholar]
- 38.Melhem NM, Talhouk R, Rachidi H, Ramia S. 2014. Hepatitis A virus in the Middle East and North Africa region: a new challenge. J Viral Hepat 21:605–615. doi: 10.1111/jvh.12282. [DOI] [PubMed] [Google Scholar]
- 39.Sdiri-Loulizi K, Hassine M, Gharbi-Khelifi H, Aouni Z, Chouchane S, Sakly N, Neji-Gue′diche M, Pothier P, Ambert-Balay K, Aouni M. 2011. Molecular detection of genogroup I sapovirus in Tunisian children suffering from acute gastroenteritis. Virus Genes 43:6–12. doi: 10.1007/s11262-011-0600-1. [DOI] [PubMed] [Google Scholar]
- 40.Federal Register. 1992. Standards for the disposal of sewage sludge. Fed Regist 503:9387–9404. [Google Scholar]
- 41.International Organization for Standardization. 2013. Microbiology of food and animal feed–horizontal method for determination of hepatitis A virus and norovirus in food using real-time RT-PCR–part 1: method for quantification. ISO/TS 15216-1:2013. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 42.Oka T, Katayama K, Hansman GS, Kageyama T, Ogawa S, Wu FT, White PA, Takeda N. 2006. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J Med Virol 78:1347–1353. doi: 10.1002/jmv.20699. [DOI] [PubMed] [Google Scholar]
- 43.Kitajima M, Oka T, Haramoto E, Katayama H, Takeda N, Katayama K, Takeda N, Katayama K, Ohgaki S. 2010. Detection and genetic analysis of human sapoviruses in river water in Japan. Appl Environ Microbiol 76:2461–2467. doi: 10.1128/AEM.02739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vrieze SI. 2012. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods 17:228–243. doi: 10.1037/a0027127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito T, Kato T, Hasegawa M, Katayama H, Ishii S, Okabe S, Sano D. 2016. Evaluation of virus reduction efficiency in wastewater treatment unit processes as a credit value in the multiple-barrier system for wastewater reclamation and reuse. J Water Health 14:879–889. doi: 10.2166/wh.2016.096. [DOI] [PubMed] [Google Scholar]
- 47.Okada M, Yamashita Y, Oseto M, Shinozaki K. 2006. The detection of human sapoviruses with universal and genogroup-specific primers. Arch Virol 151:2503–2509. doi: 10.1007/s00705-006-0820-1. [DOI] [PubMed] [Google Scholar]