Abstract
The role of vitamin D in the regulation of lung immune defense and inflammatory response has attracted more and more attention. Vitamin D deficiency is closely related to respiratory tract infections. However, few studies have elucidated the mechanism of vitamin D deficiency on host pulmonary resistance to Aspergillus fumigatus (A. fumigatus). In this paper, the role of autophagy and Treg regulation in the treatment of rat models of A. fumigatus infection with vitamin D was investigated. We intratracheally injected the A. fumigatus spores into Mice fed with sufficient vitamin D (VitD+) or deficient diets (VitD-). Mortality, fungal load and weight changes were evaluated. The conidia of lung tissue were isolated for analysis of viability. Alveolar macrophages (AMs) were stimulated with a viable A. fumigatus conidia for determining the formation of lysosomes in vitro. The autophagy-related proteins dectin-1, ROS and LC3BII expression in AMs were measured. Fluorescence and Western blot were performed to evaluate the autophagic flux and Treg cells were detected by flow cytometry. After inoculation with A. fumigatus, the vitamin D deficient mice exhibited a higher rate of death, more fungal growth, and more weight loss than its sufficient peers. The viability of A. fumigatus conidia in VitD+ mice was significantly lower than that in VitD- mice. In the case of A. fumigatus infection, vitamin D delays the formation of lysosomes against A. fumigatus through autophagy. The autophagy flow measurement experiment also found that the vitamin D group lowered autophagy levels in cells and a small number of Treg cells. In conclusion, Vitamin D deficiency can lead to impaired lung defense in mice, which may be associated with the formation of excessive autophagy-induced lysosomes and increased counts of Treg cells.
Keywords: Vitamin D, A. fumigatus, LC3BII, autophagy, Treg cells
Introduction
Vitamin D is known to be of physiological importance in calcium homeostasis and bone health [1]. Vitamin D exerts biological function through the activation of the vitamin D receptor (VDR), enhancing calcium absorption in the small intestine and promoting bone mineralization. Human endogenous vitamin D is mainly derived from the skin through adequate sun exposure, more specifically, the 7-dehydrocholesterol stored in the skin is converted to cholecalciferol (Vitamin D3) by the action of ultraviolet B-rays. Vitamin D3 is delivered to the liver through blood flow and converted to calcidiol. Studies have demonstrated that the production of active 1, 25-dihydroxyvitamin D3 (1,25D3) is catalyzed by 1α-hydroxylase, which is conventionally known to carry out in the kidneys. Without sufficient active vitamin D, the serum calcium concentration will decrease, which will lead to demineralization of the bone.
In addition to its endocrinological effects, Vitamin D has been demonstrated to play a beneficial role in pulmonary immunity and inflammatory processes [2,3]. Extra-renal 1α-hydroxylase are expressed in various cells of the immune system including airway epithelium [4], dendritic cells [5] and alveolar macrophages (AMs) [6] as well as lymphocytes [7], indicating that lungs are also important organs for the generation of active vitamin D. Localized 1,25D3 plays a role in all aspects of immune regulation. Immune effects of vitamin D involve enhanced antimicrobial peptide catholic secretion, inhibition of chemokine production and dendritic cell maturation, and alteration of T-cell differentiation [8]. The cellular effects mentioned above play a pivotal role in the host resistance against pathogens, and the lack of vitamin D will compromise its immune activity.
Accumulating evidence suggests that individuals with vitamin D deficiency are susceptible to various respiratory diseases [3]. A. fumigatus remain the most important Aspergillus [9] known to cause respiratory infections in immunocompromised patients. Vitamin D deficiency results in impaired body resistance to A. fumigatus by increasing inflammation and prolonging inflammation duration [10]. Few in-depth studies have been conducted on the ability of the host to protect against A. fumigatus infection through vitamin D.
Based on the basis that active vitamin D is generated locally in lung also it can act as an immunomodulatory molecule at this barrier site, also autophagy-related proteins are related with Candida albicans Infections [11-13]. We speculated that the impaired lung resistance to A. fumigatus infection may be attributed to vitamin D deficiency through autophagy and Treg cells. In this study, we tried to verify our assumptions by giving mice different doses of vitamin D diets and observing the extent of lung aspergillosis infection in mice.
Material and methods
Animals
All animal experiments were approved by the Animal Experimental Ethical Committee at Anhui university of science and technology under university-approved protocols. We purchased four-week old female BALB/c mice (from Anhui medical university). Mice were raised in groups of specific pathogen-free environment within small animal care facilities at Anhui university of science and technology. Mice were exposed to a 12-hr light/dark cycle and provided with assigned diet and tap water ad libitum. Weight and health status of mice were monitored weekly. For infection models, mice were anesthetized with ether.
Vitamin D-deficient diet
After a two-week acclimation, mice were fed with Vitamin D-deficient diet (AIN-93G/No vitamin D) or VitD+ diet (AIN-93G) and maintained at least four weeks. The adequate vitamin D diet contained 1000 IU of vitamin D3/kg of food. Serum concentration of 25-(OH) D3 was determined by enzyme-linked immunosorbent assay (ELISA) to confirm vitamin D deficiency.
Preparation of A. fumigatus
The A. fumigatus 42202 (ATCC) strain was cultivated at 37°C for 3 days on Sabouraud dextrose agar. The collected conidia were washed and suspended in PBS supplement with 0.1% Tween 20 (resuspension buffer) and separated from the mycelia by gauze. A. fumigatus conidia were counted microscopically and further confirmed by hemacytometer. The resuspension buffer was centrifuged to obtain a precipitate containing A. fumigatus conidia, and A. fumigatus conidia were resuspended in PBS at a concentration of 3.5 × 108 conidia/ml.
In vivo A. fumigatus infection, survival and tissue burden assessment
Mice were mildly anesthetized with ether and administered intratracheally at a concentration of 50 μl of 3-8 × 107 viable spores while maintaining an upright position. Within 1, 2 hours after inoculation, the mice recovered completely and had a healthy appearance until disease characteristics are obvious 25-30 hours after the infection. The clinical manifestations and weight changes of each group were recorded daily. The overall condition of the mice was assessed every 8 hours after A. fumigatus infection. Body weight of mice was measured regularly every morning. The rectal temperature of the mice was measured by the electronic thermometer twice a day. At the specific time after infection, lung tissue was taken out of mice, and the log10 colony-forming units (CFUs) per lung were evaluated for fungal burden.
Determination of conidia activity
Fluorescent staining are conducted according to the recommendation of L13152 live/dead BaclightTM Bacterial viability kits, fluorescent dyes are added to the conidia, then the conidia were cultured at 37°C for 60 min and washed with PBS for 5 times, the conidia are observed by fluorescence microscopy for the analysis of viability. The live/dead BaclightTM Bacterial viability kits consist of two dyes, one is SYTO9 green-fluorescent nucleic acid stain, and the other is the red-fluorescent nucleic acid stain, propidium iodide. The use of the SYTO9 dye alone can stain all conidia, and when both dyes are used at the same time, SYTO9 fluorescence is attenuated by the infusion of propidium iodide through the damaged cell membrane.
Isolation and stimulation of AMs
Surgical exposure was performed to separate the trachea. After a 20-gauge needle was inserted into the trachea, alveolar macrophages were collected from the lung tissue with pre-cooled PBS (10 times). For stimulation assays, bronchoalveolar lavage fluid (BALF) was collected and centrifuged at 4°C, 300 g for 8 min. The precipitated cells were suspended in RPMI 1640 containing penicillin (100 units/ml), streptomycin (100 mg/ml), and 5% fetal bovine serum to prepare a cell suspension at a concentration of 2 × 106/ml. 250 μl (5 × 105 cells) was added to each well of the 24-well plate and incubated at 37°C in a humidified atmosphere of 5% CO2 for 2 hours to promote adhesion to the cell walls. After washing the plates with RPMI 1640 three times, the adherent cells were gently scraped with a curette and then fresh RPMI 1640 containing 10% FBS was added. Alveolar macrophages could be cultured separately in the culture medium or co-cultured with viable A. fumigatus conidia. The ratio of conidia to cells was 1:10 (MOI = 0.1).
Real-time PCR
Total RNA was extracted from cultured cells or lung tissue using Trizol reagent (Invitrogen Life Technologies), and cDNA was synthesized using the M-MLV reverse transcriptase kit (Promega) according to manufacturer’s instructions. The detection of the target gene was carried out on the CFX96 real-time system (Bio-Rad). Gene-specific primers and probes for beta-actin, LC3BII, dectin-1 and ROS were purchased. Data were expressed using the delta-delta Ct method.
Western blot analysis
The cultured cells were treated with RIPA lysis buffer (Genechem) to isolate the total protein. The extracted protein was subjected to 8-18% SDS-PAGE gradient gel electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). The membranes were incubated with the following primary antibodies at 4°C overnight after blocked with TBST solution containing 5% BSA for 2 hours. The primary antibodies used in the experiment included the following: anti-dectin-1 at 1:1000 (Santa Cruz Biotechnology), anti-ROS at 1:1000 (Abcam), LC3BII at 1:1000 (Sigma) and β-actin at 1:2000 (Sigma). After washing three times in TBST solution, the membranes were incubated with the respective secondary antibodies (1:2000, Cell Signaling) for 1 h at room temperature. Protein bands were visualized using the TMB (Cell Signaling company). The mean pixel density of each protein band was quantified using Quantity One software 4.6.2 (Bio-Rad). β-actin expression were used as internal controls.
Autophagic flow detection
The stably expressed GFP-LC3 RAW264.7 cells from Anhui University of Science And Technology were infected by A. fumigatus with or without vitamin D. The expression of autophagy related protein LC3BII was observed by fluorescence. LC3BII showed green fluorescent spots. The ratio of the LC3BII level of the chloroquine treatment group to the corresponding untreated group in the C was used as an indicator of the autophagic flow rate.
Flow cytometry analysis
Splenic cells were harvested and counted by flow cytometry. Cellular population were washed and resuspended at a concentration of 0.5 × 106 to 1 × 106 PBS buffer plus 0.1% NaN3, and Fc receptors were blocked by the addition of unlabeled anti-CD16/32 (Fc blocking, eBioscience Inc.). After Fc receptor blocking, immunostaining for cell surface molecules was performed for 30 min at 4°C. Cells were washed twice with PBS buffer. The data acquired were analyzed with Cyt Expert software. Fluorochrome-conjugated antibodies directed against the following antigens were obtained: CD4-FITC, CD8a-PC5.5, CD25-APC, CD19-PC7, CD45-APC-Alexa Fluor700 and FOXP3-PE (Bio1egend, USA). Foxp3/Transcription Factor Fixation/Permeabilization Concentrate and Diluent Kit (eBioscience Inc.). Numbers of relevant cell types were determined by combining flow cytometry data.
ELISA
For in vivo experiments, animals were sacrificed and their blood was clotted on ice for 10 minutes, and serum was separated by centrifugation at 4°C and was stored at -80°C for further analysis of cytokine levels. Serum levels were measured in duplicate wells using mouse IL-10, IFN-γ, TGF-β and IL-4 ELISA kit (Dakewe Biotech, Beijing, China). All assays were performed according to the manufacturer’s protocol.
Statistical analysis
Statistical analysis was performed with the GraphPad Prism 5.0 software. Survival rate differences were analyzed using the Log-rank and Wilcoxon tests. Paired data were analyzed by paired samples t-test or Mann-Whitney test for non-parametric data. P<0.05 were considered statistically significant. All experiments were repeated three times.
Results
Vitamin D deficiency defected host resistance to A. fumigatus
In this research, we used BALB/c mice to study whether vitamin D deficiency changed the host resistance to A. fumigatus. After 8 weeks of feeding, VitD- mice had significantly lower 25-(OH)D3 levels compared to VitD+ mice (Figure 1, P<0.01). Serum calcium and phosphorus concentrations were similar over the eight weeks period (data not show). No significant changes were observed in average weight on VitD- and VitD+ group at eight weeks (Figure 1). Mice were infected with A. fumigatus conidia by airway to assess mortality, lung fungus load and body weight changes.
Figure 1.
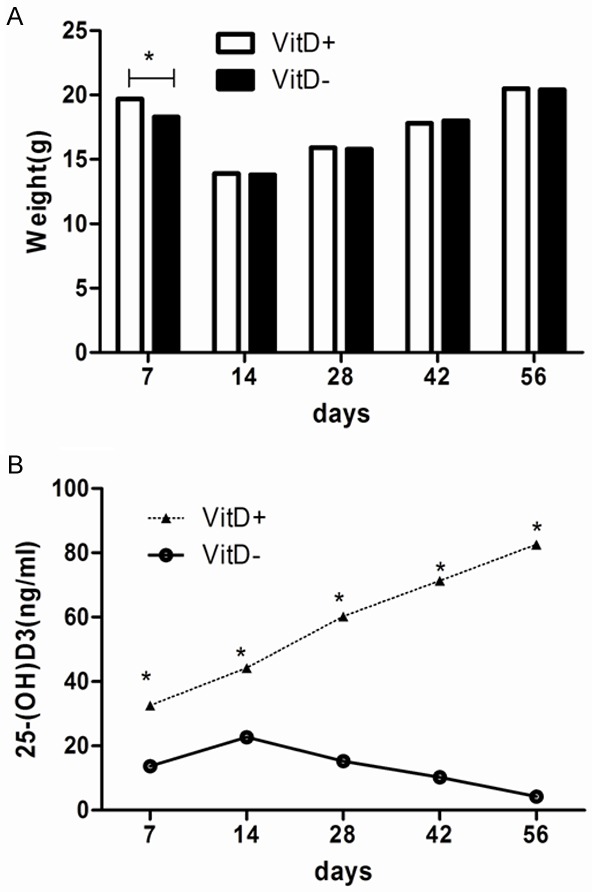
Serum concentrations of Vitamin D and weights of mice in the two dietary groups. BALB/c mice were fed with VitD+ and VitD- diets after weaning. Three mice from each group were sacrificed. Blood was drawn from the retro-orbital venous plexus and the serum concentration of 25-(OH)D3 (ng/ml) was measured every two weeks. Data represents mean ± SD (n = 3, repeated three times each). *P<0.01 (student’s t-test).
The mortality of VitD- mice infected with 5 × 107 conidia was significantly higher than that of VitD+ mice (36.7%; 11/30 mice versus 13.3%; 4/30 mice respectively) (Figure 2A).When the amount of conidia increased to 7 × 107, the mortality rate of VitD- mice reached half by 5 days (Figure 2B). When 3 × 107 conidia were inoculated, no deaths occurred in either of the two groups within 28 days (data not shown). Pulmonary resistance to A. fumigatus was compromised due to vitamin D deficiency. The fungal load in the lungs of VitD- mice was significantly higher than that of VitD+ mice at 24, 48 and 72 hrs after 3 × 107 conidia inoculation (Figure 2C). VitD- mice were more likely to lose body weight when stimulated with 3 × 107 conidia, and VitD- mice gained much less weight than VitD+ mice (Figure 2D).
Figure 2.
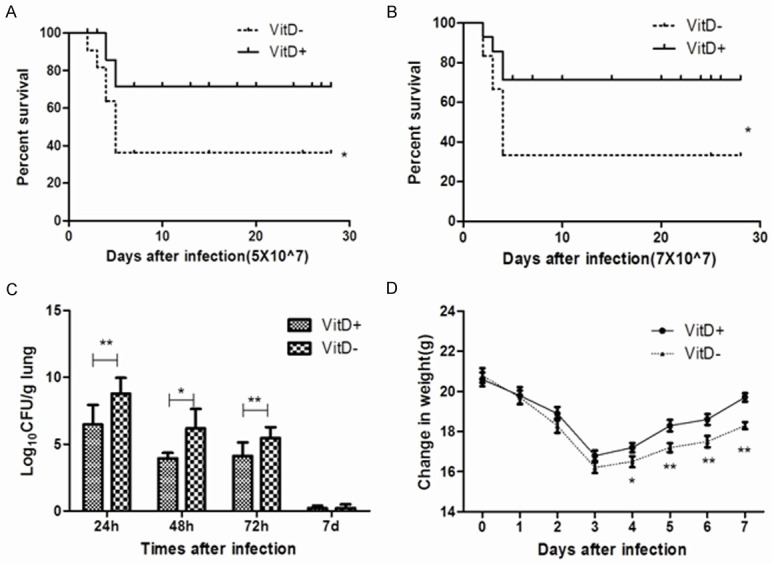
Increased susceptibility of VitD- mice after infected with A. fumigatus. 40 μl of 5 × 107 (A), or 7 × 107 (B) A. fumigatus conidia were injected intratracheally into VitD+ and VitD- mice. The survival status of mice was monitored continuously for 28 days and expressed as fraction of survival. These two graphs showed the cumulative results of three independent experiments for each inoculum dose. 0.05 was the significance level for log rank test and Wilcoxon test. (C) Pulmonary fungal loads were observed in VitD+ and VitD mice after intratracheal infection with 3 × 107 A. fumigatus conidia. Mice lung tissue homogenates were filtered and plated to measure CFU. Values represent as log10 of CFU average values from three independent studies (three for each dilution using three mice of each group). *P<0.05 and **P<0.01 (Mann-Whitney U-test) were set as a statistically significant judgment level. (D) The mice weight changes induced by the infection were monitored for 7 days after intratracheal infected with 3 × 107 A. fumigatus conidia. Results are expressed as means ± SD (n = 15). *P<0.05, **P<0.01 (Student’s t-test).
VitD+ and VitD- mice were put to death 3 days after intratracheal inoculation of 3 × 107 A. fumigatus conidia. Then we isolated and lyzed the lung tissues, the homogenates were filtered through gauze to get the conidia, the conidia were stained with Annexin Syto9/PI for the analysis of viability through the fluorescence microscope. The viability of A. fumigatus conidia was significantly lower in VitD+ mice (Figure 3D) than in VitD- mice (Figure 3C).
Figure 3.
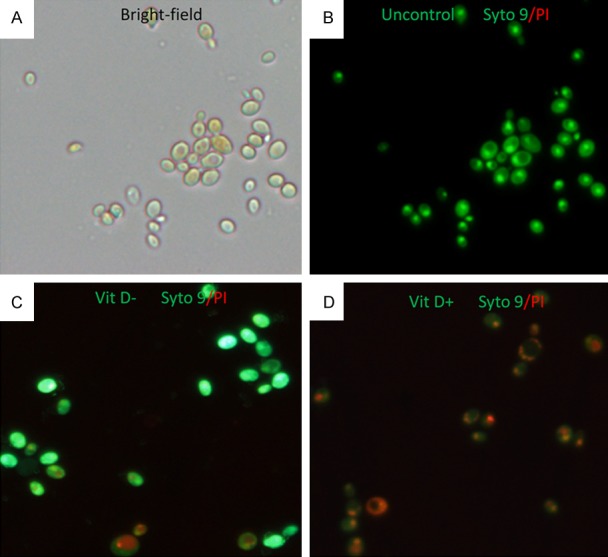
The inhibitory effect of vitamin D on the viability of A. fumigatus conidia was observed by fluorescence microscopy. A. the viability of A. fumigatus conidia was observed in bright field. B. negative control (A. fumigatus conidia was stained with Annexin Syto9/PI). C, D. The lung tissues from VitD-/VitD+ mice were lyzed three days post intratracheal challenge with 3 × 107 A. fumigatus conidia, the Annexin Syto9 (green)/PI (red)-stained conidia (magnification 400 ×) was shown above to distinguish dead or alive conidia (the SYTO9 generally labels all the conidia, in contrast, PI penetrates only conidia with damaged membranes).
The above evidence suggested that vitamin D deficiency impaired the host’s ability to resist A. fumigatus.
Vitamin D delays the formation of lysosomes against A. fumigatus
The AMs were obtained from lung tissue from BALB/c mice, then the cells are cultured with or without Vitamin D, after challenged with 3 × 107 A. fumigatus conidia at 1 hr or 3 hrs, the conidia were stained with Hochest33342/Lyso-Tracker Red for the analysis of the formation of lysosomes, by 1 hr, phagocytosis of the conidia was observed in VitD- AMs instead of VitD+ AMs, by 3 hr, the conidia were completely in the VitD- AMs (Figure 4A). In contrast, phagocytosis of the conidia began to appear in VitD+ AMs. In VitD- AMs, aggregation of lysosomes was more apparent, by 3 hrs, lysosome colocated with the conidia, while in VitD+ AMs, lysosomes were scattered in the cytoplasm (Figure 4B).
Figure 4.
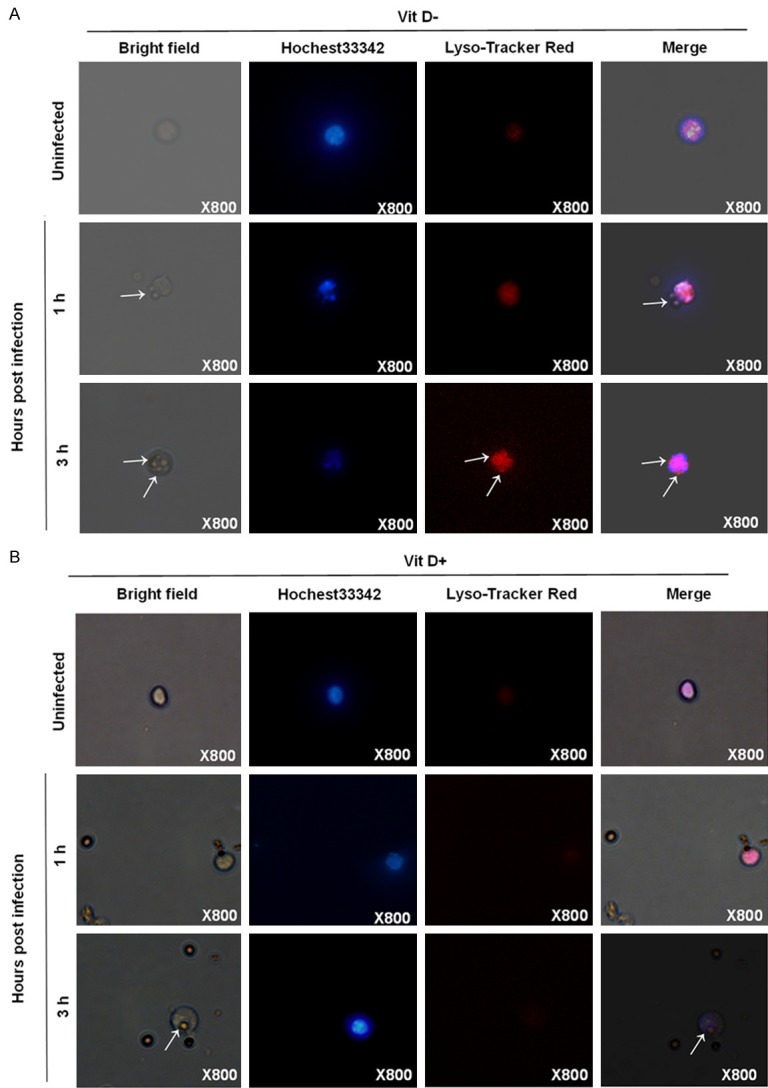
The inhibitory effect of vitamin D on the formation of lysosomes against A. fumigatus. The AM cells from the lung tissues of BALB/c mice were infected by A. fumigatus. with or without vitamin D, the AMs (magnification 800 ×) stained with Hochest33342/Lyso-Tracker Red for the analysis of the formation of lysosomes are shown at the indicated times.
Vitamin D deficiency may compromise pulmonary resistance to A. fumigatus through autophagy
To determine whether autophagy occurs when AMs were challenged with A. fumigatus, the autophagy-related proteins were analyzed. Figure 5A showed that dectin-1, ROS and LC3BII increased gradually beginning at 0-12 h. p.i in VitD+ AMs, while these autophagy-related proteins were at a higher level continuously in VitD- AMs. The autophagy-related proteins reached a peak value in VitD+ AMs (12 h) earlier than in VitD- AMs (48 h).
Figure 5.
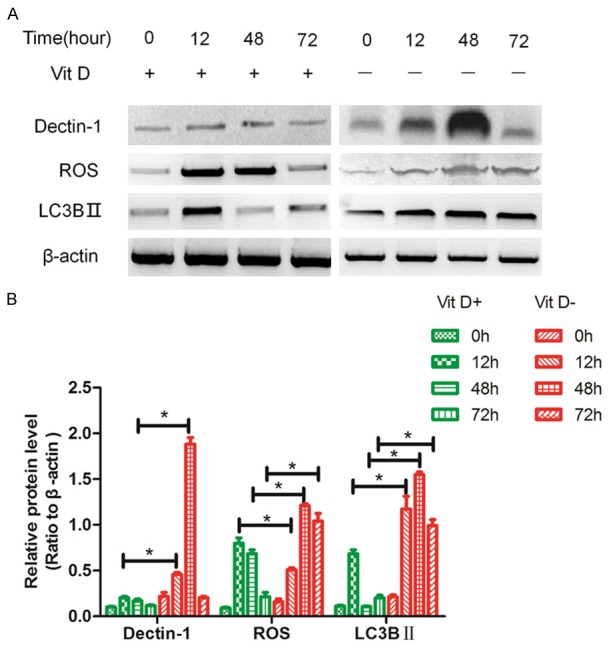
The effect of vitamin D on the autophagy-ralated proteins. The AM cells from the lung tissues of BALB/c mice were infected by A. fumigatus with or without vitamin D, the protein was extracted at specific time p.i., expression of dectin-1, ROS and LC3BII were analyzed by Western blotting. Data represents mean ± S.E.M. (n = 3, repeated three times each). *P<0.05 (Student’s t-test).
From the results of the autophagy flow test (Figure 6), we found that the levels of autophagy in the vitamin D treated group were lower and statistically significant than those in the untreated group.
Figure 6.
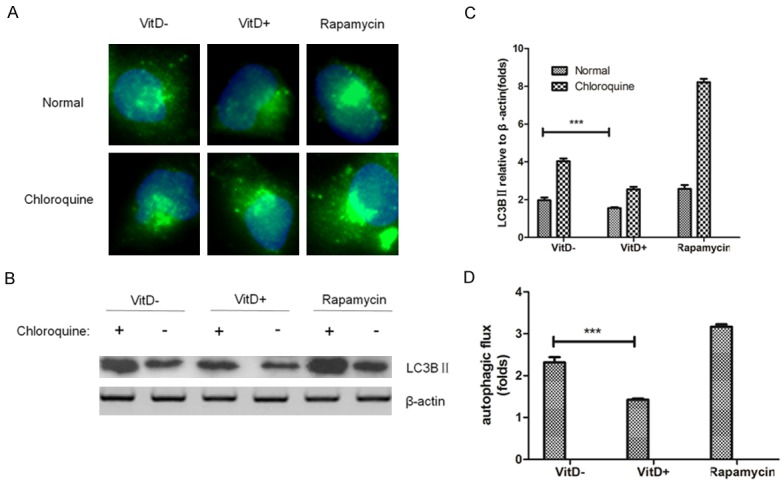
The effect of vitamin D on the autophagic flux. Fluorescence and Western blot were performed to evaluate the autophagic flux on stably expressed GFP-LC3BII and infected by A. fumigatus RAW264.7 cells treated with or without vitamin D for 6 h. A: The expression of autophagy related protein LC3BII was observed by fluorescence. LC3BII showed green fluorescent spots. Vitamin D group was found to significantly reduce the number of autophagic bodies. B, C: LC3BII protein levels were analyzed by Western blot. D: The ratio of the LC3BII level of the chloroquine treatment group to the corresponding untreated group in the C was used as an indicator of the autophagic flow rate, in which the vitamin D group significantly inhibited the level of autophagic flow. Data represents mean ± S.E.M. (n = 3, repeated three times each). ***P<0.001 (Student’s t-test).
These demonstrated that Vitamin D can maintain cellular low levels of efficient autophagy in the early stages of lung clearance of Aspergillus fumigatus and prevent damage by excessive autophagy.
Treg cells increased in A. fumigatus-challenged VitD- mice
To determine whether immune response against A. fumigatus-challenged VitD+/- mice. Splenic cells were analyzed by flow cytometry to examine the percentage of Th1, Th2 and Treg cells. As shown in Figure 7A, the levels of Foxp3 expression were much higher in Vit D- group than those in the VitD+ group. While there was no significant difference between Th1 and Th2 cells (date no shown). We also found that the levels of IL-10 and TGF-β in the vitamin D treated group were lower and statistically significant than those in the untreated group (Figure 7B). Meanwhile, there were no differences between the two groups in the production of IL-4 and IFN-γ.
Figure 7.
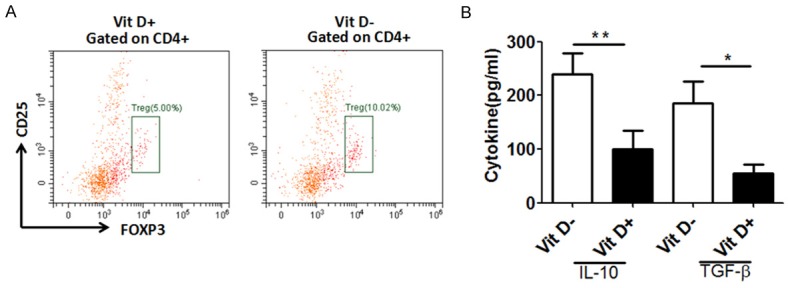
Treg cells increased in A. fumigatus-challenged VitD- mice. A. BALB/c mice were fed with VitD+ and VitD- diets after weaning. Splenic cells were harvested and gated on lymphocytes and aggregates (scatter area versus height). Representative dot plot examples from one mouse with frequencies of CD25+FOXP3+ cells among CD4+ lymphocytes. B. BALB/c mice were fed with VitD+ and VitD- diets after weaning. Three mice from each group were sacrificed. Blood was drawn from the retro-orbital venous plexus and the serum concentration of 25-(OH)D3 (ng/ml) was measured at the end of the experiment. Data represents mean ± SD. Meanwhile, there were no differences between the Vit D+ group and the Vit D- group in the production of IL-6 and IFN-α. Data are means ± SD. Asterisk indicates statistical significance between Vit D- groups and Vit D+ group: *P<0.05, **P<0.01. Results from 1 of 3 independent experiments are presented.
Discussion
Our results demonstrated that the survival rate of VitD- mice infected with A. fumigatus decreased coupled with the impaired lung clearance of organisms. Vitamin D showed an inhibitory effect on the viability of A. fumigatus conidia and reduced the number of Treg cells in vivo. In vitro, experiments showed that vitamin D inhibits the formation of lysosomes against A. fumigatus. Vitamin D deficiency may compromise pulmonary resistance to A. fumigatus through autophagy. In vivo and in vitro experiments confirmed that vitamin D can maintain cellular low levels of efficient autophagy and a small number of Treg cells in the early stages of lung clearance of A. fumigatus and prevent damage by excessive autophagy.
Our survival experiments showed that VitD- mice had higher mortality rate and impaired fungal clearance, which were similar with previous study [10]. An important finding from this study is that the viability of A. fumigatus conidia was significantly lower in VitD+ mice than in VitD- mice in vivo. Seasonal fluctuations in respiratory infections have been observed in population biology. The seasonal variation in vitamin D levels [14] has been proposed to explain this phenomenon. In addition to its endocrine function, Vitamin D helps to promote innate immunity [15]. A. fumigatus infection can affect the function of T helper cells and effect phagocytic cells [16], meanwhile vitamin D can also act on T, B and Treg cells, macrophages, cytokines and chemokine release, reactive nitrogen, and oxygen species, dectin-1, toll-like and mannose receptors. Treg mediated cellular immunity plays a crucial role in immune homeostasis during the infection phase. In our study, Treg cell is increased in VitD- group, which can secrete regulatory cytokines, such as IL-10, TGF-β. Due to its important role in differentiation and functional effects. Treg plays a negative regulatory role in CD4+T cell mediated responses due to its ability to inhibit the Th1-mediated inflammatory response and Th2-mediated allergic response. Since TGF-β is closely related to the differentiation of Treg, we proposed that enhanced autophagy could be the reason why the Treg cells increased in A. fumigatus-challenged VitD- group. This also has been shown to correlate directly with increased Treg-related cytokine TGF-β level. Treg cells play an essential role in the development of protective immunity to the ubiquitous mold A. fumigatus by sufficient Vit D supplements.
All of these findings inspired us to study the relationship between vitamin D and A. fumigatus infection. It has been reported that cathelicidin induced by calcitriol through IL-37 [17] can delay the growth of Mycobacterium tuberculosis in vitro, which may be a cause of lower serum vitamin D levels in tuberculosis patients [18]. The data in our study suggested that vitamin D may boost the host immunity and enhance the resistance to A. fumigatus.
Pattern recognition receptors (PRRs), such as C-type lectin receptors (CLRs), like Dectin-1 and Toll-like receptors (TLRs), are closely related to the host’s ability to identify fungi in a timely and effective manner. Antigen-presenting cells (APCs) and phagocytic cells are responsible for phagocytosis and neutralization of pathogens, and subsequently phagocytosed pathogens are processed and degraded in organellites known as phagosomes. Autophagy is an intracellular catabolic pathway that transfers cytoplasmic material to lysosomes, which is characterized by the formation of double membrane vesicles called autophagosomes. Traditionally, autophagy has been demonstrated to play a pivotal role in maintaining cell metabolism and homeostasis [13]. Recently, a growing number of reports suggested that autophagy was involved in the elimination of fungal by the host [11,12,19,20]. Autophagy has a dual effect on the regulation of cell death and mild autophagy protects cells from harmful conditions to promote cell survival. Severe and rapid autophagy will induce programmed cell death, known as autophagy-mediated cell death [21-23]. Our experiments showed that vitamin D inhibits the formation of lysosomes, which may maintain moderate autophagy, it will be helpful to reduce programmed cell death and enhance sustained antifungal ability, thus protect cells from A. fumigatus. to promote cell survival.
In conclusion, we have found that vitamin D plays an important role in host immunization against A. fumigatus in immunocompetent hosts. Vitamin D enhances the host’s cellular immune function against A. fumigatus by regulating the autophagy and Treg cells involved in A. fumigatus infection. These studies provide the basis for clarifying the mechanisms by which vitamin D is responsible for the risk of Invasive Pulmonary Aspergillosis (IPA) in immunosuppressed patients. The study of autophagy in the body after infection with A. fumigatus is still at the initial stage. We should devote more efforts to study how to make these autophagic proteins play a role in protecting the host from A. fumigatus infection.
Acknowledgements
We thank the Anhui University of Science and Technology, and Huai’an First People’s Hospital laboratory staffs for providing the clinical specimens, animals, and data for this study. All the experiments were performed at the Anhui University of Science and Technology and data analysis was completed in Huai’an First People’s Hospital. The experiments were supported by the key program of social development research in Huai’an [HAS2015009].
Disclosure of conflict of interest
None.
References
- 1.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 2.Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158:20–25. doi: 10.1111/j.1365-2249.2009.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansdottir S, Monick MM. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm. 2011;86:217–237. doi: 10.1016/B978-0-12-386960-9.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 6.Adams JS, Gacad MA. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med. 1985;161:755–765. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 8.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Dagenais TR, Keller NP. Pathogenesis of aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Xu X, Cao E, Yu B, Li W, Fan M, Huang M, Shi L, Zeng R, Su X, Shi Y. Vitamin D deficiency causes defective resistance to Aspergillus fumigatus in mice via aggravated and sustained inflammation. PLoS One. 2014;9:e99805. doi: 10.1371/journal.pone.0099805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyrmizi I, Gresnigt MS, Akoumianaki T, Samonis G, Sidiropoulos P, Boumpas D, Netea MG, van de Veerdonk FL, Kontoyiannis DP, Chamilos G. Corticosteroids block autophagy protein recruitment in aspergillus fumigatus phagosomes via targeting dectin-1/Syk kinase signaling. J Immunol. 2013;191:1287–1299. doi: 10.4049/jimmunol.1300132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, Gresnigt MS, Begun J, Plantinga TS, Joosten LA, van der Meer JW, Chamilos G, Netea MG, Xavier RJ, Dinarello CA, Romani L, van de Veerdonk FL. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci U S A. 2014;111:3526–3531. doi: 10.1073/pnas.1322831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez J, Malireddi RK, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan JL, Tan H, Peng J, Kanneganti TD, Virgin HW, Green DR. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol. 2015;17:893–906. doi: 10.1038/ncb3192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–2432. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeke F, Gysemans C, Korf H, Mathieu C. Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol. 2010;25:1597–1606. doi: 10.1007/s00467-010-1452-y. [DOI] [PubMed] [Google Scholar]
- 16.Sirivoranankul C, Martinez M, Chen V, Clemons KV, Stevens DA. Vitamin D and experimental invasive aspergillosis. Med Mycol. 2014;52:847–852. doi: 10.1093/mmy/myu048. [DOI] [PubMed] [Google Scholar]
- 17.White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76:3837–3843. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato E, Imafuku S, Ishii K, Itoh R, Chou B, Soejima T, Nakayama J, Hiromatsu K. Vitamin D-dependent cathelicidin inhibits Mycobacterium marinum infection in human monocytic cells. J Dermatol Sci. 2013;70:166–172. doi: 10.1016/j.jdermsci.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Kanayama M, Inoue M, Danzaki K, Hammer G, He YW, Shinohara ML. Autophagy enhances NFkappaB activity in specific tissue macrophages by sequestering A20 to boost antifungal immunity. Nat Commun. 2015;6:5779. doi: 10.1038/ncomms6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J, Becker C, Lowell CA, Underhill DM. Dectin-1-triggered recruitment of light chain 3 protein to phagosomes facilitates major histocompatibility complex class II presentation of fungal-derived antigens. J Biol Chem. 2012;287:34149–34156. doi: 10.1074/jbc.M112.382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryter SW, Mizumura K, Choi AM. The impact of autophagy on cell death modalities. Int J Cell Biol. 2014;2014:502676. doi: 10.1155/2014/502676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jing K, Lim K. Why is autophagy important in human diseases? Exp Mol Med. 2012;44:69–72. doi: 10.3858/emm.2012.44.2.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol. 2011;21:387–392. doi: 10.1016/j.tcb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


