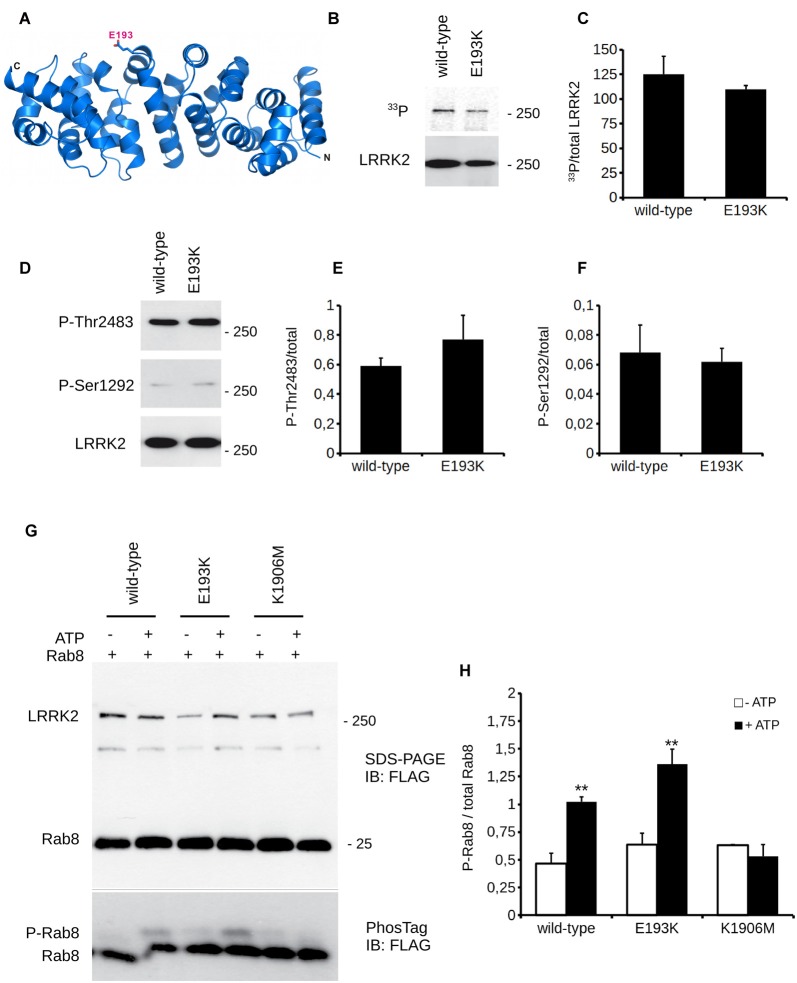
Figure 2.
E193K variant does not affect LRRK2 kinase activity. (A) Ribbon diagram showing a structural homology model (based on the ARM domain of adenomatous polyposis coli (APC) protein from human, PDB: 3T7U) for the LRRK2 N-terminal region (residues 7-322). N-terminus (N) and C-terminus (C) of the structural model as well as the position of E193 (stick model) are indicated. (B) In vitro radioactive kinase assays of 3x-Flag LRRK2 WT and E193K purified from HEK cells. Five nanomolar of purified Flag-LRRK2 WT or E193K from HEK293T cells were subjected to in vitro radiometric kinase assays and the radioactivity incorporated was quantified by PhosphoImager. Upper panel represents autophosphorylation and lower panel western blotting with anti-flag antibodies to quantify total loading. (C) Quantification of moles of 33P incorporated by LRRK2. Graphs report mean ± standard error (SE), n = 4. (D) In vitro kinase assays as in (A). Five nanomolar of purified Flag-LRRK2 WT or E193K from HEK293T cells were also subjected to in vitro non-radioactive kinase assays. Autosphosphorylation levels were measured by western blotting with anti-Ser1292 and anti-Thr2483 antibodies (upper panels). Lower panel represents total protein loading, probed with anti-flag antibodies. (E) Quantification of phosphorylation at Thr2483 and (F) Ser1292, expressed as optical density and normalized vs. total LRRK2 protein amount. Graphs report mean ± SE, n = 4. (G) Purified Flag-LRRK2 WT, E193K or K1906M protein were incubated with Flag-Rab8 (1:15 molar ratio) in the presence or absence of 1 mM ATP and subjected to PhosTag assay to analyze phosphorylation stoichiometry and SDS-PAGE to verify total protein amount. Anti-Flag antibody was used to reveal LRRK2 and Rab8. (H) Quantification of Rab8 phosphorylation, expressed as optical density and normalized vs. total Rab8 (phosphorylated + unphosphorylated band). Data are presented as mean ± SE (n = 3); ** p < 0.01 vs. -ATP, same LRRK2 variant.