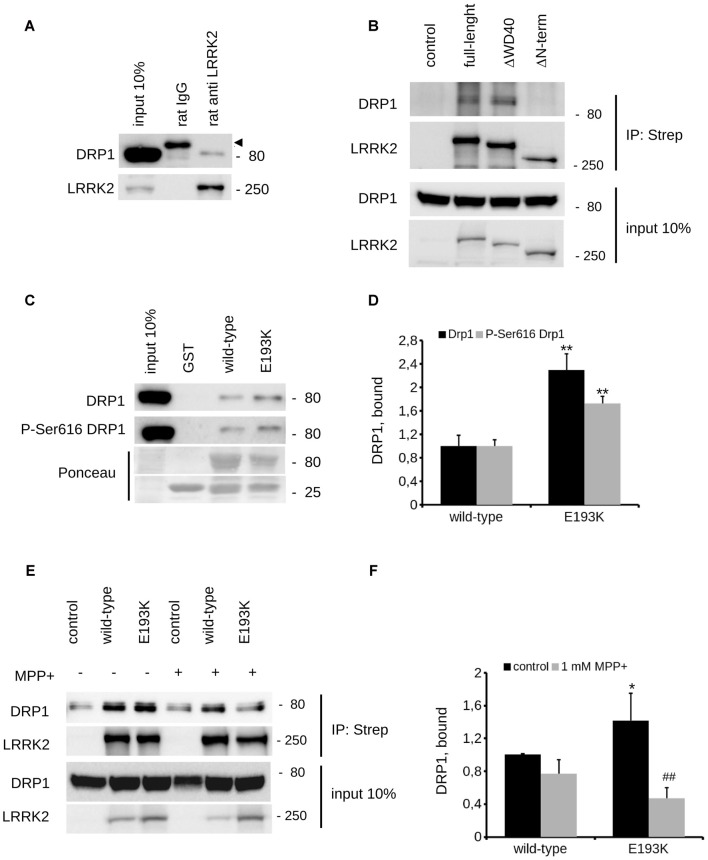
Figure 7.
E193K variant affects LRRK2-DRP1 complex. (A) Extracts of mouse adult forebrain were incubated with anti-LRRK2 antibodies or rat IgG. The immunocomplexes were isolated with protein G-Sepharose and the samples were resolved by SDS-PAGE and analyzed by immunoblotting with anti DRP1 and anti LRRK2 antibodies. Arrowhead indicates unspecific band recognized by anti-rabbit secondary antibody. (B) We isolated on streptavidin resin strep-FLAG-LRRK2 full-length (full-length), strep-FLAG-LRRK2ΔWD40 (ΔWD40) and strep-FLAG-LRRK2ΔN–terminal (ΔN–terminal) protein from HEK293 over-expressing cells. Interacting proteins were resolved by western-blotting. (C) We performed a GST-pull down approach to explore the interactome associated to LRRK2 N-terminal Armadillo domain. GST-fusion proteins corresponding to Armadillo domain of LRRK2 WT and LRRK2 E193K (E193K) were used to retain interactors from adult forebrain lysate. The complexes were isolated with GSH-Sepharose beads, the samples were resolved by SDS-PAGE and analyzed by immunoblotting with anti DRP1 and anti P-Ser616 DRP1 antibodies. (D) We evaluated the extent of DRP1 and P-Ser616 DRP1 bound to WT and E193K Armadillo domain expressed as ratio over WT domain. Graph reports mean ± SE; n = 4; **p < 0.01 vs. WT. (E) N2A cells expressing Strep-FLAG-LRRK2 WT or Strep-FLAG-LRRK2 E193K variant were treated or not with MPP+ (1 mM, 24 h) solubilized and processed for streptavidin immunopurification. We evaluated the extent of DRP1 binding to LRRK2 by measuring the amount of DRP1 protein co-precipitating with Strep-FLAG LRRK2 variant. (F) The graph reports the amount of DRP1 recovered in FLAG immunoprecipitates. Data were normalized to the amount of LRRK2 variant immunoprecipitated and expressed as mean ± SE, n = 7; *p < 0.05 vs. WT, ##p < 0.01 vs. E193K not treated.