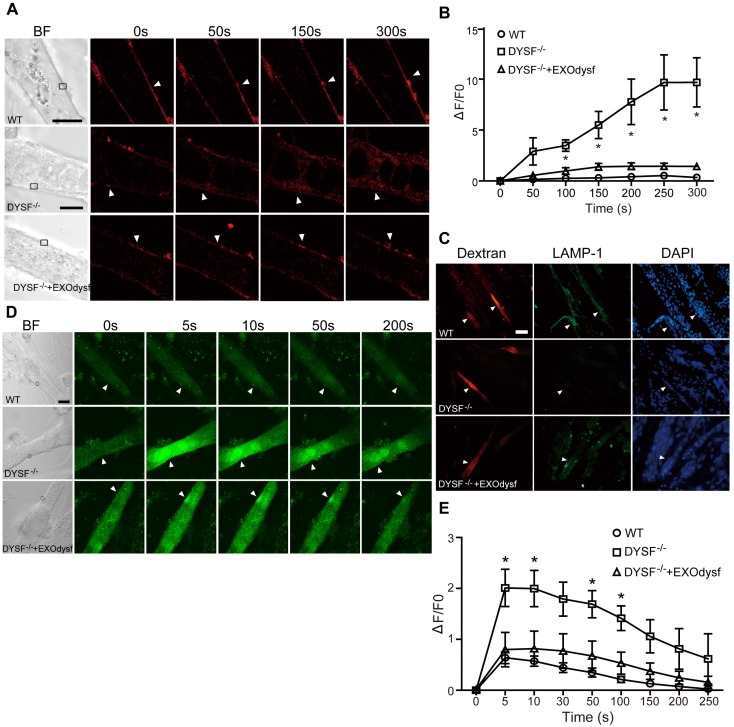
Figure 3.
Functional restoration of EXOdysf-treated murine DYSF-/- myotubes. (A) Two-photon confocal microscopy images showing the membrane repair capacity of EXOdysf in DYSF-/- myotubes. Arrowheads point to the laser injury sites. The FM4-64 dye (red) was used to show the integrity of the membrane (scale bar = 10 μm). WT: wild-type; BF: bright field. (B) Quantitative analysis of fluorescence intensity at different time-points in WT (circles), DYSF-/- (squares) or EXOdysf-treated DYSF-/- (triangles) myotubes. Significant reductions in fluorescence intensity were achieved in EXOdysf-treated DYSF-/- myotubes compared to untreated myotubes (n=15, two-tailed test, *P<0.05). (C) Immunostaining of dysferlin and lysosome-associated membrane protein-1 (LAMP-1) in EXOdysf-treated DYSF-/- myotubes. Nuclei were counterstained with DAPI (blue). Arrowheads point to the scrape wounding sites (scale bar =100 μm). (D) Two-photon confocal microscopy images showing the Ca2+ influx in EXOdysf-treated DYSF-/- myotubes. Arrowheads point to the laser injury sites (scale bar = 10 μm). (E)Quantitative analysis of fluorescence intensity at different time-points in WT (circles), DYSF-/- (squares) or EXOdysf-treated DYSF-/- (triangles) myotubes. Significant reductions in fluorescence intensity were achieved in EXOdysf-treated DYSF-/- myotubes compared to untreated myotubes at earlier time-points (n=15, two-tailed test, *P<0.05).