Figure 8.
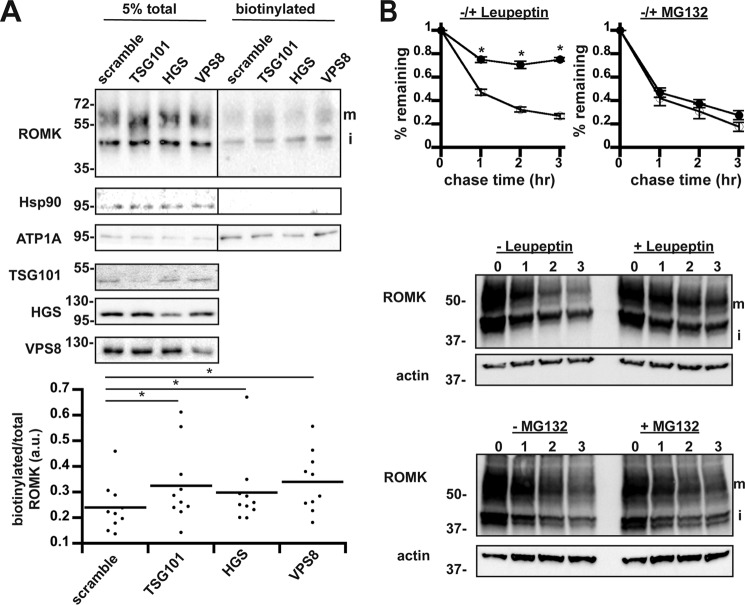
Depletion of select ESCRT and CORVET components increases ROMK surface expression in HEK293 cells. A, lysates from HEK293 cells expressing ROMK under the control of the doxycycline-inducible “T-REx” promoter were examined by anti-ROMK immunoblot in toto (left) or were surface biotinylated and precipitated with NeutrAvidin beads (right). ROMK migrates as two bands: an immature (i) ER-glycosylated band at ∼45 kDa and a larger mature (m) glycosylated species of ∼55–65 kDa (75). All blots were stripped and reprobed for cytosolic Hsp90 and plasma membrane-enriched ATP1A to show accurate loading and selective biotinylation of only cell-surface proteins. An empty lane between the total and biotinylated samples was cropped out as indicated by the line. Quantitation of change in cell-surface ROMK normalized to total protein for each replicate is shown below. Cell lysates from all siRNA-targeted factors were blotted on separate membranes to show efficient knockdown. Silencing for nine experimental replicates led to a decrease of 63 ± 4.7% for TSG101, 71 ± 4.3% for HGS, and 55 ± 5.1% for VPS8. *, p < 0.05 (Student's t test). B, HEK293 cells expressing ROMK under the control of the doxycycline-inducible “T-REx” promoter were pretreated with the lysosomal protease inhibitor leupeptin or MG132 (closed circles) or vehicle (open circles) for 1 h prior to cycloheximide addition. Samples were harvested at the indicated times. ROMK expression was assessed by Western blotting and normalized to the initial time point. For this figure, only the upper mature band (m) was quantitated. Representative blots are shown, and all blots were stripped and reprobed for actin as a loading control. Data represent the means of three independent experiments. Error bars show standard errors of the mean, and * indicates a significant (p < 0.05) difference as assessed by Student's t test.