Figure 3.
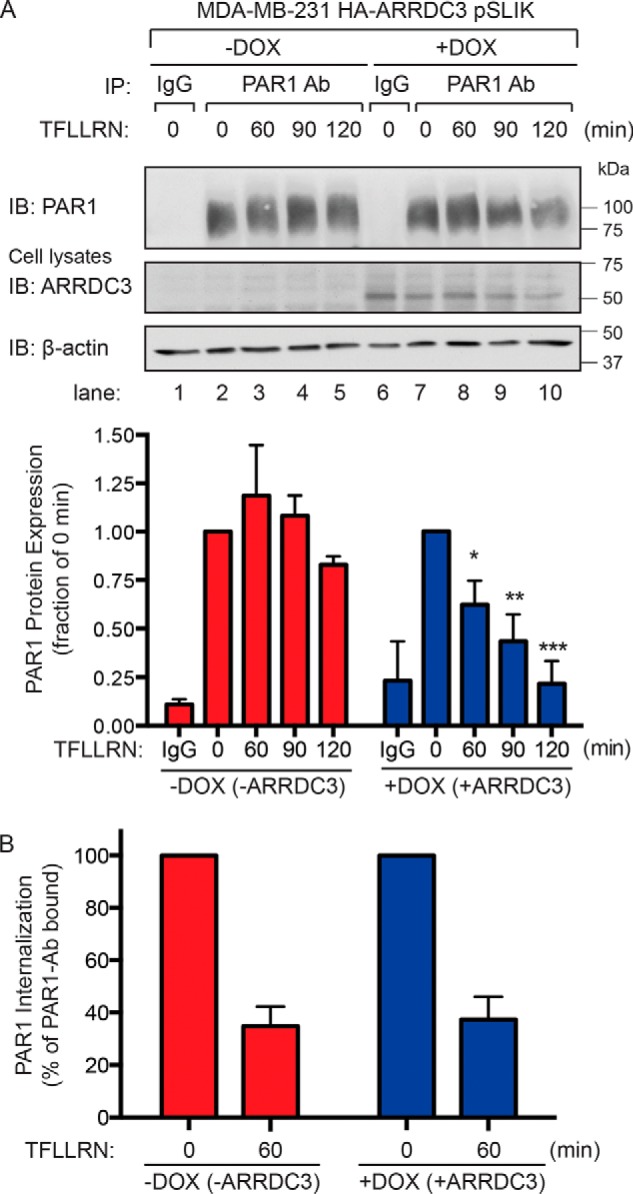
Agonist peptide–induced PAR1 lysosomal degradation is restored in cells re-expressing ARRDC3. A, MDA-MB-231 HA-ARRDC3 pSLIK cells were treated with or without 1 μg/ml DOX for 48 h. Cells were then stimulated with 100 μm TFLLRN peptide agonist for the indicated times, lysed, and immunoprecipitated using the anti-PAR1 WEDE antibody or anti-IgG antibody as a control. Immunoprecipitates were immunoblotted with anti-PAR1 rabbit polyclonal antibody. Cell lysates were immunoblotted with anti-HA antibody to detect ARRDC3. β-Actin expression was determined as a control. The data shown (mean ± S.D. (error bars), n = 3) were quantified by densitometry and represented as PAR1 expression relative to unstimulated control (0 min). Statistical significance was determined by one-way ANOVA (*, p < 0.05; **, p < 0.01; ***, p < 0.001; n = 3). B, MDA-MB-231 HA-ARRDC3 pSLIK cells were treated with or without 1 μg/ml DOX for 48 h, labeled with anti-PAR1 WEDE antibody at 4 °C, and then stimulated with or without 100 μm TFLLRN for the indicated times. Cells were then fixed, and the amount of cell-surface PAR1 was determined by ELISA. The data (mean ± S.D., n = 3) are represented as the percentage of PAR1 remaining on the cell surface relative to untreated control (0 min) and representative of three independent experiments.
