Figure 9.
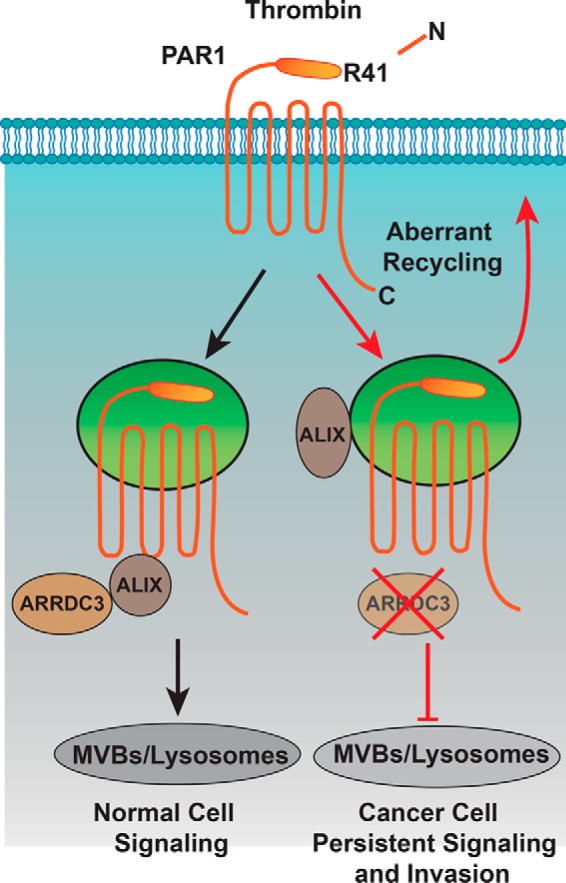
Model of ARRDC3 and PAR1 trafficking. Thrombin binds to and cleaves the PAR1 N terminus at arginine 41, exposing a new N-terminal domain that acts like a tethered ligand. Due to the irreversible proteolytic mechanism of PAR1 activation, internalization and lysosomal sorting is critical for termination of G protein signaling. Unlike most classic GPCRs, activated PAR1 is rapidly sorted from endosomes to lysosomes through a non-canonical pathway mediated by ARRDC3 and ALIX. In invasive breast cancer, activated PAR1 is internalized and recycled and fails to sort to lysosomes for degradation and consequently signals persistently, which promotes tumor cell invasion and growth. We discovered that loss of ARRDC3 of expression in invasive breast cancer is responsible for defective PAR1 trafficking that results in persistent signaling and cellular invasion.
