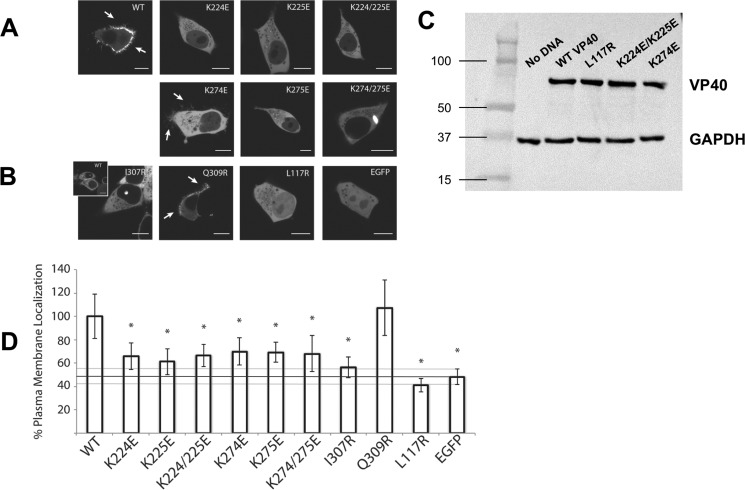
Figure 6.
Confocal live cell imaging of EGFP-VP40 and respective mutations. HEK293 cells were transfected with the indicated mutant constructs and imaged on a Zeiss LSM 710 confocal microscope 22–24 h post-transfection. A, WT EGFP-VP40 exhibited strong localization at the plasma membrane with extensive membrane protrusions (white arrows). CTD-1 mutants (K224E, K225E, and K224E/K225E) were mainly diffusely localized in the cytoplasm with no detectable VLP formation at the PM. Likewise, charge reversal mutations in the CTD-2 (K274E, K275E, K274E/K275E) resulted in little to no detectable PM localization. However, the mutant K274E demonstrated a partial ability to form VLPs as evidenced by the fractured protrusion at the PM (white arrows). Additionally, the double mutant K274/K275E formed bright intracellular globules. B, structural mutants (I307R and L117R) were also not detected at the PM but remained predominantly cytosolic with L117R diffuse in the nucleus as well. However, I307R occasionally exhibited a unique punctate nuclear localization similar to WT (inset). The control Q309R, which is near I307R but does not spontaneously induce octameric ring formation (1), displayed WT-like enrichment at the PM and VLP formation (white arrows). C, Western blot confirming equivalent heterologous expression of various VP40 mutants in cell culture. Western blot for GAPDH is shown as a loading control. D, quantification of %PM localization of WT and all mutants (%PM localization = PM intensity/(PM + cytosol intensity)·100%). Between 16 and 25 cells were analyzed for each mutant from three independent experiments. All bars represent the mean ± S.E. and are normalized to WT. A Student's t test was calculated for all mutants with respect to WT (*, p < 0.005). The %PM localization of EGFP is indicated with a black line as a marker for baseline PM localization. Scale bars, 10 μm.