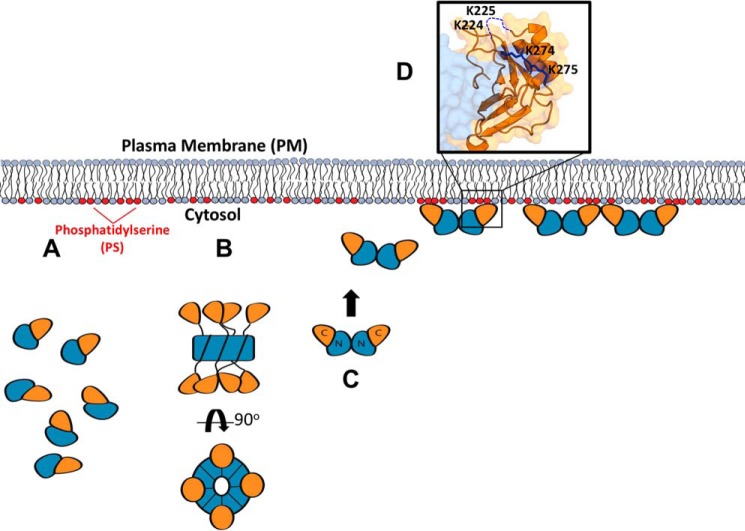
Figure 9.
Summary of VP40 structures in the life cycle of EBOV. As described throughout, VP40 forms a monomer, dimer, and RNA-binding octamer. A, monomer is the simplest unit of VP40 but may not possess biologically relevant lipid-binding properties. Despite harboring high affinity for PS-containing membranes, mutations of the NTD α-helical interface that mediate dimerization disrupt PM localization of VP40 as described herein as well as in Bornholdt et al. (1). VP40 trafficking to the PM may require the intact dimer to interact with host proteins such as Sec24C. B, octameric ring conformation renders VP40 less fit for PS binding, suggesting alternative cellular functions consistent with its proposed role in regulation of viral transcription (1). Presumably, the PS-binding surface in the VP40 CTD is not optimized for high-affinity PS binding in the octameric form. Additionally, the octameric VP40 may not be sufficient for proper trafficking to the PM. C, VP40 dimers harbor the requisite PS-binding properties for PM localization and budding. D, selective PS binding is mediated through CTD residues Lys224, Lys225, Lys274, and Lys275 in VP40 dimers.