Figure 5.
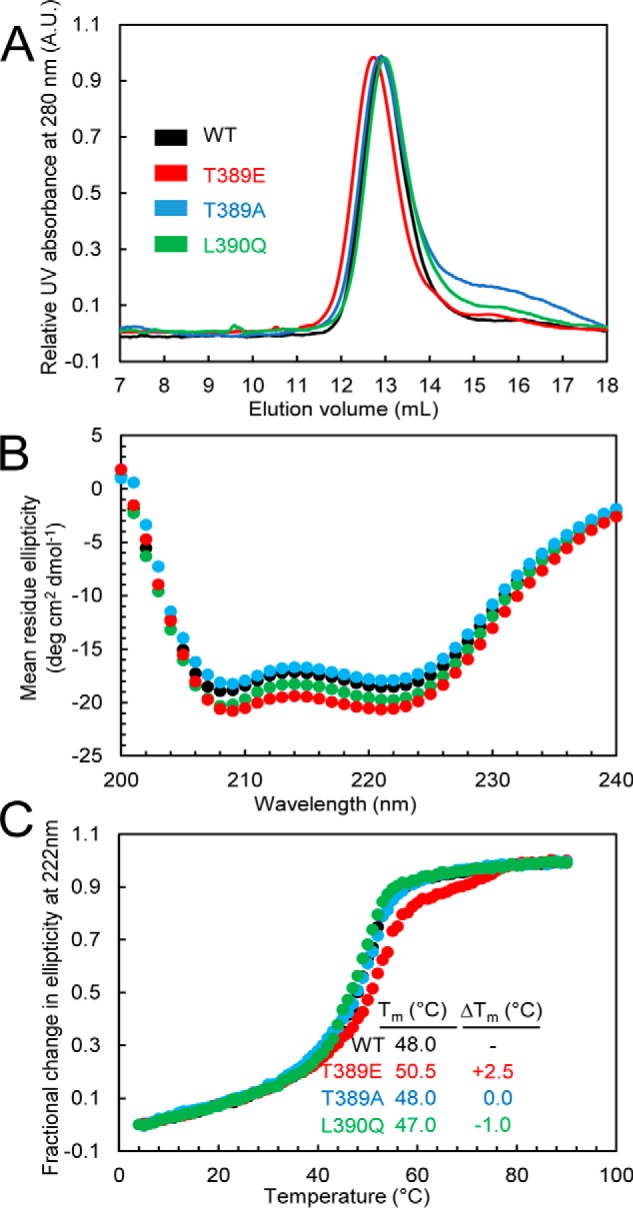
A, SEC of the STIM1 sequence 234–491 in wildtype STIM1 and the T389A-, T389E-, or L390Q-STIM1 mutant. Apparent molecular masses of WT, T389E, T389A, and L390Q were 2.4, 2.5, 2.4, and 2.3× the theoretical monomeric molecular mass of STIM1(234–491) (30.8 kDa), consistent with the well-established notion that the cytosolic domains of STIM1 recapitulate the dimeric nature of full-length STIM1 when expressed in live mammalian cells. Data are representative of at least two distinct protein preparations. B, secondary structure obtained by far-UV CD spectroscopy for the STIM1 sequence 234–491 in wildtype STIM1 and the T389A, T389E, or L390Q mutant. Far-UV CD spectra of STIM1(234–491) were acquired at 0.3–0.5 mg ml−1. Data are representative of at least two distinct protein preparations. C, thermal stability analyses by changes in far-UV CD spectroscopy at 222 nm for the STIM1 sequence 234–491 in wildtype STIM1 and the T389E-, T389A-, or L390Q-STIM1 mutants. Thermal stability was measured at protein concentrations of 0.3–0.5 mg ml−1. The apparent Tm values were 48.0, 50.5, 48.0, and 47.0 °C for the wildtype, T389E, T389A, and L390Q proteins, respectively. Data are representative of at least two distinct protein preparations. A.U., absorbance units.
