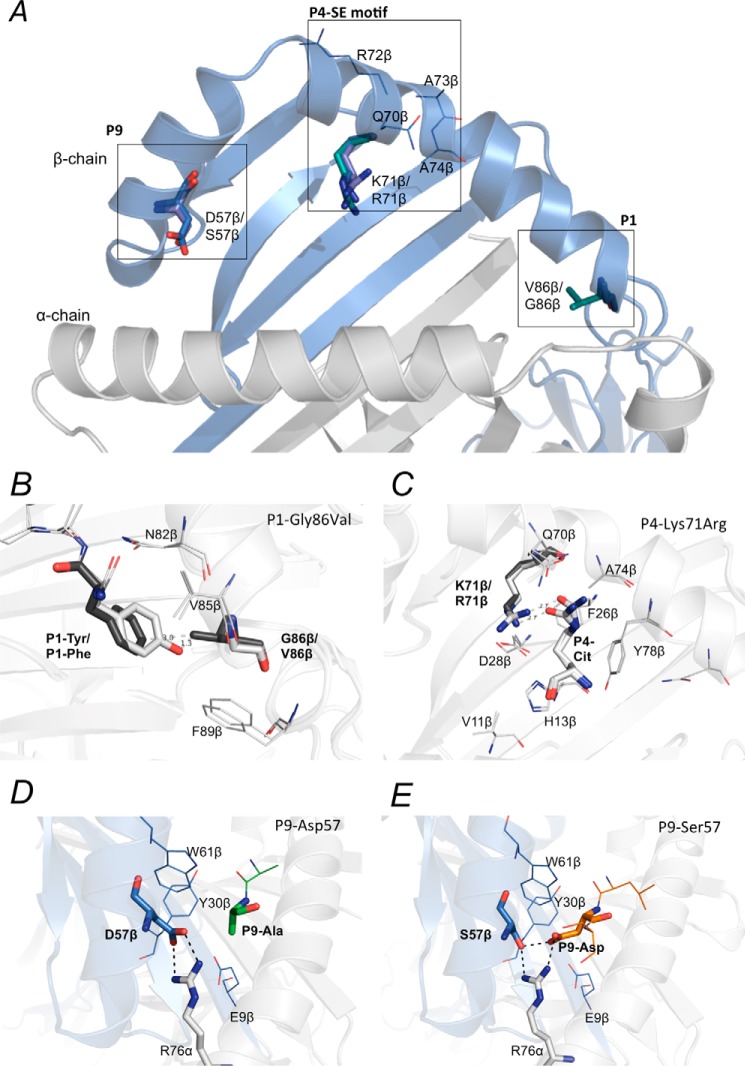
Figure 3.
Polymorphic residues in the peptide-binding cleft of HLA-SE molecules. Polymorphic residues in the P1, P4, and the P9 pocket of HLA-DRB1*04:01, DRB1*04:04, DRB1*04:05, and the shared-epitope motif are shown (A). The G86V variation on the DRβ chain of HLA-DRB1*04:04 may obstruct binding of hydrophobic residues such as Tyr in the P1 pocket (B). The P4-Cit forms a conserved hydrogen bond with the Lys-71β/Arg-71β, a polymorphic residue in the P4 pocket between HLA-DRB1*04:01 and DRB1*04:04/*04:05 (C). The P9 pocket of HLA-DRB1*04:01 and DRB1*04:04 consists of a Asp-57β that shapes the P9 pocket of these two alleles by forming a salt bridge with the conserved Arg-76α (D). The D57S variation on the DRβ chain of HLA-DRB1*04:05 forms extensive hydrogen bonds with P9-Asp in the peptide (E). The P9 pocket of HLA-DRB1*04:05 with a Ser-57β that has a shorter side chain has been shown to have a preference for negatively charged residue in the P9 position of peptides.