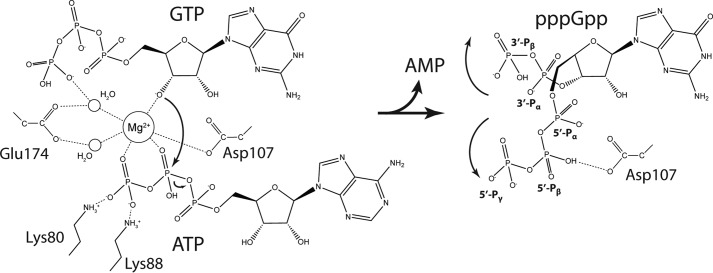
Figure 5.
Mechanism of (p)ppGpp synthesis by SAS enzymes. Detailed catalytic mechanism for (p)ppGpp synthesis by SAS enzymes based on the structures of the pre-catalytic (left) and post-catalytic (right) state structures of SaRelP. In the pre-catalytic state (left), a Mg2+ ion is held firmly in place by tight octahedral coordination facilitated by the β- and λ-phosphate oxygen atoms of ATP, two water molecules (which again are constrained by both carboxylate oxygen atoms of Glu-174), one carboxylate oxygen from Asp-107, and the 3′-OH group of GTP. The electrophilic metal ion promotes deprotonation of the 3′-OH group on GTP and subsequently a nucleophilic attack (large, curved arrow) of the resulting alkoxide ion onto the β-phosphate group of ATP causing hydrolysis of the phosphate ester between the α- and β-phosphate groups (small, curved arrow). The developing negative charge of the pentavalent transition state is stabilized by the combined positive charge of two arginine residues and six lysine residues in the neighborhood (not all shown). In the post-catalytic state (right), the 5′ β- and λ-phosphate groups of pppGpp flip over and the λ-phosphate group assumes the position of the λ-phosphate group of ATP in the pre-catalytic state, stabilized through hydrogen bonding with Asp-107. Likewise, the new 3′ α- and β-phosphate groups shift position to generate the U-shaped conformation of pppGpp found in chain A of the post-catalytic state.