Abstract
Lactic acidosis is a commonly observed clinical condition that is associated with a poor prognosis, especially in malignancies. We describe a case of an 81-year-old patient who presented with symptoms of tachypnea and general discomfort. Arterial blood gas analysis showed a high anion gap acidosis with a lactate level of 9.5 mmol/L with respiratory compensation. CT scanning showed no signs of pulmonary embolism or other causes of impaired tissue oxygenation. Despite treatment with sodium bicarbonate, the patient developed an adrenalin-resistant cardiac arrest, most likely caused by the acidosis. Autopsy revealed Gleason score 5 + 5 metastatic prostate cancer as the most probable cause of the lactic acidosis. Next-generation sequencing indicated a nonsense mutation in the TP53 gene (887delA) and an activating mutation in the PIK3CA gene (1634A>G) as candidate molecular drivers. This case demonstrates the prevalence and clinical relevance of metabolic reprogramming, frequently referred to as “the Warburg effect,” in patients with prostate cancer.
Keywords: Mutation, p53 mutations, PIK3CA mutations, PTEN mutations, IDH1 mutations, Prostate cancer, Lactic acidosis, Warburg effect, Metabolic reprogramming, Case report
Introduction
Lactic acidosis is a clinical condition that frequently occurs in patients admitted to the department of internal medicine. It results from systemic accumulation of lactate and protons due to increased conversion of pyruvate to lactate by the enzyme lactate dehydrogenase (LDH). Clinically, the causes of lactic acidosis are divided into two categories [1]. First, cases in which impaired tissue oxygenation is the principal cause, for example due to reduced tissue perfusion by sepsis or atherosclerosis. These cases are classified as lactic acidosis “type A.” Certain commonly prescribed drugs, including metformin, salicylates, β2-agonists, and propofol, are another cause of systemically elevated lactate levels. They predominantly increase the lactate production through interference with oxidative phosphorylation in the tricarboxylic acid cycle in mitochondria. Decreased clearance of lactate has been described in fulminant liver diseases. Cancer is another described cause of lactic acidosis. These cases of diverse pathogenesis are categorized as “type B.”
Tumor cells consume large quantities of glucose as can be seen on fluorodeoxyglucose-F18 (FDG)-PET scans. Despite sufficient amounts of oxygen and nutrients, they preferentially metabolize glucose through glycolysis, which yields high lactate levels. The preference of cancer cells to metabolize glucose through glycolysis was initially discovered by the German physiologist Otto Warburg. Although a rather inefficient way to generate ATP, this pathway has been proposed to primarily facilitate rapid incorporation of nutrients into tumor tissue (Fig. 1). The numerous glycolytic intermediates of this pathway can be converted in lipids, nucleotides, and proteins that are essential constituents of cancer cells [2]. Large-scale genomics analysis of cancer tissues by “The Cancer Genome Atlas” (TCGA) and other consortia have revealed widespread alterations in gene expression that may facilitate “the Warburg effect.” Hypoxia-inducible factors (HIF) are assumed to drive these changes in gene expression [2].
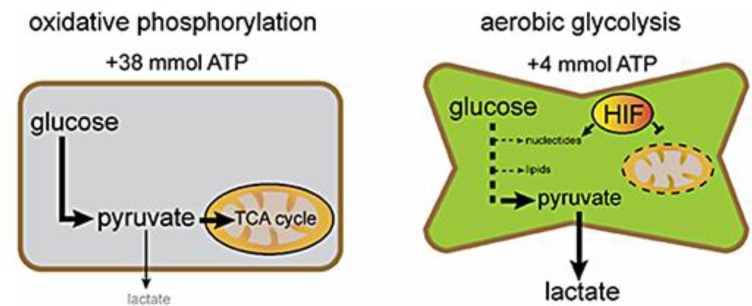
Fig. 1.
The differences in glucose metabolism between normal and cancerous tissues are shown. Oxidative phosphorylation in the tricarboxylic acid (TCA) cycle, which yields large quantities of ATP, is the dominant metabolic pathway in differentiated cells. Cancer cells, in contrast, preferentially use glycolysis, which is assumed to be driven by hypoxia-inducible factors (HIF) and yields numerous precursors of cell constituents, but limited amounts of ATP.
Prostate cancer is a leading cause of death in the Western world. It is the second most common type of cancer in men with an expected number of 161,360 new cases per year in the United States. With the prostate-specific antigen (PSA) as readily available serologic biomarker, the disease is increasingly diagnosed as localized disease. The histopathologic Gleason score is currently the best prognostic biomarker for survival at diagnosis [3]. A minority of 17.6% presents with Gleason score ≥8, which confers a risk of developing metastatic disease [4]. Common alterations in prostate cancer genomes are fusions of androgen-regulated promoters with E26 transformation-specific (ETS) transcription factors [5]. These alterations most likely render patients susceptible to hormone therapy, which is currently standard first-line treatment. In addition to alterations in the androgen signaling cascade, lesions have been detected in the PI3K, MAPK, and DNA repair pathways, which may be critical for disease progression [5]. Few reports have appeared on the expression of genes involved in glucose metabolism in prostate cancer.
Case Report
We here present a case of an 81-year-old male patient who was referred to our outpatient clinic. His medical history describes an ischemic stroke, hypertension with a stable stage 3 nephropathy, and successfully treated multiple myeloma type IgA lambda. Two years before presentation, he suffered from symptomatic anemia, caused by a monoclonal gammaglobulinemia (29 g/L). He received treatment with melphalan 10 mg, prednisone 100 mg, and bortezomib 1.3 mg/m2 for 9 cycles, which resulted in a significant decrease of the paraproteinemia and a complete recovery of the anemia. Due to disease progression, he subsequently started with treatment consisting of lenalidomide 15 mg and dexamethasone 40 mg, which was poorly tolerated and stopped after 2 cycles. Subsequently, the patient was in a clinically stable condition with a low paraproteinemia (1–5 g/L) and mild anemia (hemoglobin 6.8 mmol/L).
In August 2015, the patient was admitted to the Department of Internal Medicine for complaints of dyspnea, general discomfort, and muscle weakness. Physical examination revealed a poor performance status (WHO 3–4), tachypnea (24/min) with a normal body temperature and blood pressure, and no clinical signs of pneumonia or cardiac decompensation. General blood tests, along with arterial blood gas analysis, were performed, which revealed a respiratory compensated metabolic acidosis (Table 1; day 0). The anion gap, corrected for the hypoalbuminemia, was found to be increased (16.8) with an elevated arterial lactate level (9.5 mmol/L). We concluded that the patient's symptoms of dyspnea, muscle weakness, and general discomfort were most likely a consequence of the lactic acidosis.
Table 1.
Metabolic detoriation: progressive lactic acidosis
| Day 0 | Day 1 | Day 2 | Normal | |
|---|---|---|---|---|
| pH | 7.37 | 7.36 | 7.23 | 7.38 to 7.46 |
| pCO2, mm Hg | 15.4 | 14.8 | 18.2 | 34.0 to 45.0 |
| Bicarbonate, mmol/L | 8.8 | 8.1 | 7.4 | 22.2 to 27.4 |
| pO2, mm Hg | 65 | 68 | 75 | - |
| O2 saturation, % | 93.1 | 93.1 | 92.7 | - |
| Base excess, mmol/L | −15.0 | −16.0 | −18.7 | −1.9 to 3.2 |
| Lactate, mmol/L | 9.5 | 12.0 | 13.6 | <2.5 |
| Chloride, mmol/L | 121 | 121 | 123 | 102 to 110 |
| Sodium, mmol/L | 142 | 144 | 148 | 136 to 145 |
| Potassium, mmol/L | 4.6 | 4.9 | 4.9 | 3.5 to 4.4 |
| Albumin, g/L | 25.3 | 26.1 | NA | 35 to 52 |
| Anion gap, mmol/L | 16.8 | 17.4 | - | 5 to 11 |
In order to differentiate between a type A and B lactate acidosis and find a potential cause, more investigations were performed. Laboratory tests and CT scanning were conducted. No signs of ischemia were observed with normal vital parameters, a normal ECG, and contrast enhancement of all major abdominal and thoracic vessels. No signs of pulmonary embolism were present. General blood tests revealed a normal liver function, a stable kidney function (eGFR 41 mL/min) and an elevated PSA level (240 µg/L; normal <4.0 µg/L). Medication (metformin, salicylates) and food supplements were discussed with the patient and no toxic cause was identified. CT imaging of the thorax and abdomen revealed an enlarged prostate, hydronephrosis of the right kidney, 2 small nodules in the liver (segment 1 and 7), and enlarged mediastinal and paratracheal lymph nodes as well as sclerotic bone metastases. We concluded that the patient suffered from type B lactic acidosis most likely caused by metastatic prostate cancer. Treatment was started with cyproterone acetate 100 mg twice daily, prednisone 65 mg once daily, and thiamine 100 mg. Treatment with chemotherapy was considered, but impossible due to poor performance status, comorbidities, and age.
Soon after diagnosis, the patient clinically deteriorated. Arterial blood gas analysis showed increasing lactate levels up to 13.6 mmol/L and lowering pH levels to 7.23 (Table 1; day 1–2). Since the patient became unconscious, he was admitted to the intensive care unit (ICU) and received 300 mmol NaHCO3 8.4%. Two days after admission to the ICU, the patient developed an adrenaline-resistant cardiac arrest. Since the patient signed a do not resuscitate (DNR) will, resuscitation did not take place.
Autopsy was performed, which revealed locally advanced and metastatic prostate cancer (Gleason score 5 + 5 = 10) with encasement of the right ureter. Histopathologic examination demonstrated metastases in the thoracic and lumbar spine, liver, lungs, and peritoneum. Furthermore, bilateral tumor thrombi were detected in the microcirculation of both lungs. No signs of activity of multiple myeloma or cardiac ischemia were noted.
On DNA isolated from the prostate carcinoma, targeted next-generation sequencing was performed aimed at a panel of genes including TP53, PIK3CA, PTEN, and IDH1 genes. A frameshift mutation was detected in exon 8 of the TP53 gene (c.887delA, p.H296Pfs*49) leading to a truncated gene product. Furthermore, an activating mutation was found in exon 10 of the PIK3CA gene (c.1634A>G, p.E545G). No mutations were observed in exon 1, 3, 5, 6, 7, and 8 of the PTEN gene and exon 4 of the IDH1 gene.
Discussion
We here present an 81-year-old male patient who presented with a pronounced lactic acidosis. Autopsy revealed metastatic prostate cancer as the most probable cause. Other contributing factors may have been the anemia, multiple myeloma, or pulmonary tumor thrombi found at autopsy. Since the patient's multiple myeloma was in a stable phase, we consider it to be a minor contributing factor to lactic acidosis. CT scanning revealed normal contrast enhancement of all major thoracic vessels at the time of presentation, with no ischemic lung segments visible. In addition, we consider the microthrombi not to be of hemodynamic significance and therefore not the principal cause of the increased lactate levels at presentation. After admission to the ICU, the patient developed rising lactate levels resulting in severe metabolic acidosis which impaired the cardiac contractility and imposed a risk of cardiac arrhythmias [6, 7]. In conclusion, we think that our patient died from a cardiac arrest caused by a symptomatic lactic acidosis caused by “the Warburg effect.”
Lactic acidosis is frequently observed in the internal medicine practice. It is commonly caused by anaerobic metabolism of glucose in hypoxic tissues. In this clinical condition, lactic acidosis may be seen as a sign of severe atherosclerosis or cardiac failure. Likewise, in other conditions with a reduced tissue perfusion, such as (septic) shock, lactic acidosis is marked as a poor prognostic biomarker. Malignancies have been recognized as alternative cause of elevated systemic lactate levels. Several mechanisms may play a role in the pathogenesis of patients with this disease. Elevated lactate levels are predominantly recycled in the liver through gluconeogenesis. It has been speculated that extensive liver metastasis may contribute to lactate acidosis through a reduced capacity of gluconeogenesis [8]. Increased production of lactate by tumor cells may also contribute to the development of lactic acidosis. Active renal lactate secretion (10–20% of the systemic lactate) does not compensate for this increased production and decreased hepatic clearance of lactate.
Analysis of gene expression in primary prostate cancer specimens has not frequently revealed “Warburg-like” profiles. A small subset (2.7%) of tumors was found to harbor mutations in the isocitrate dehydrogenase 1 (IDH1) gene [5]. Inactivation of this mitochondrial enzyme was shown to reduce oxidative phosphorylation through suppression of pyruvate dehydrogenase (PDH) and stimulation of hypoxia-inducible factor 1 (HIF1) [9]. Another subset of tumors with particular high histopathologic Gleason scores was shown to contain mutations in the PTEN and TP53 tumor suppressor genes [5]. Combined loss was found to induce the Warburg effect in prostate cancer xenografts through upregulation of hexokinase 2 [10]. To investigate the molecular basis of this case, targeted sequencing of these genes was performed on DNA isolated from tumor tissue. While no mutations were detected in PTEN and IDH1, critical mutations in TP53 and PIK3CA were found. Similar to PTEN loss, the substitution of glutamic acid (E) at position 545 of the PIK3CA gene product to glycine (G) results in increased catalytic activity and enhanced signaling of the phosphoinositide 3-kinase (PI3K) pathway. In conclusion, we speculate that the Warburg effect in tumor cells of the patient described here may have been mediated by mutations in the TP53 and PIK3CA genes.
The treatment of lactic acidosis aims to eliminate the underlying cause. Treatment of lactic acidosis with NaHCO3 has been subject of debate in the medical community, since it may induce accumulation of CO2 in tissues, promote intracellular acidosis, and thereby potentially worsen outcome [11]. Others proposed that sodium bicarbonate treatment may increase the blood pH and thereby improve the response to inotropic agents such as adrenaline and improve hemodynamic stability. Two small independent randomized controlled trials have been conducted and showed little impact of NaHCO3 on cardiac function and hemodynamic parameters as measured in the ICU, despite an increasing pH [12, 13]. Since our patient had progressive acidosis, he received NaHCO3 as supportive care, which did not significantly affect cardiovascular parameters and could not prevent his cardiac arrest. Dichloroacetate, which decreases lactate production through activation of PDH, has been described as alternative treatment. It has been investigated in a randomized controlled clinical trial, but did not improve the survival of patients with lactic acidosis [14].
Since the elucidation of the signal transduction pathways leading to the Warburg effect, novel targeted small molecules have been developed, such as SR9243. SR9243 interacts with the nuclear liver X receptor (LXR) and selectively targets lipogenesis, and the Warburg effect in cancer cells [15]. Agents like SR9243 have presumably also clinically a “broad-spectrum” anti-tumor activity, are currently subject of preclinical research, and may become part of cancer treatment regimens [15]. We hope that in the near future, agents like SR9243 can be used to treat both the malignancy as well as the lactic acidosis in (critically ill) patients with cancer.
Statement of Ethics
The family of the patient has given informed consent for the autopsy, including the pathology studies. These studies complied with the institute's ethics committee rulings.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1.Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014;371:2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andren O, Fall K, Franzen L, Andersson SO, Johansson JE, Rubin MA. How well does the Gleason score predict prostate cancer death? A 20-year followup of a population based cohort in Sweden. J Urol. 2006;175:1337–1340. doi: 10.1016/S0022-5347(05)00734-2. [DOI] [PubMed] [Google Scholar]
- 4.Penney KL, Stampfer MJ, Jahn JL, Sinnott JA, Flavin R, Rider JR, et al. Gleason grade progression is uncommon. Cancer Res. 2013;73:5163–5168. doi: 10.1158/0008-5472.CAN-13-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990;258:C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- 7.Marsh JD, Margolis TI, Kim D. Mechanism of diminished contractile response to catecholamines during acidosis. Am J Physiol. 1988;254:H20–H27. doi: 10.1152/ajpheart.1988.254.1.H20. [DOI] [PubMed] [Google Scholar]
- 8.Record CO, Iles RA, Cohen RD, Williams R. Acid-base and metabolic disturbances in fulminant hepatic failure. Gut. 1975;16:144–149. doi: 10.1136/gut.16.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izquierdo-Garcia JL, Viswanath P, Eriksson P, Cai L, Radoul M, Chaumeil MM, et al. IDH1 mutation induces reprogramming of pyruvate metabolism. Cancer Res. 2015;75:2999–3009. doi: 10.1158/0008-5472.CAN-15-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Xiong H, Wu F, Zhang Y, Wang J, Zhao L, et al. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014;8:1461–1474. doi: 10.1016/j.celrep.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Son YK, An WS. Effect of sodium bicarbonate administration on mortality in patients with lactic acidosis: a retrospective analysis. PLoS One. 2013;8:e65283. doi: 10.1371/journal.pone.0065283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper DJ, Walley KR, Wiggs BR, Russell JA. Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. A prospective, controlled clinical study. Ann Intern Med. 1990;112:492–498. doi: 10.7326/0003-4819-112-7-492. [DOI] [PubMed] [Google Scholar]
- 13.Mathieu D, Neviere R, Billard V, Fleyfel M, Wattel F. Effects of bicarbonate therapy on hemodynamics and tissue oxygenation in patients with lactic acidosis: a prospective, controlled clinical study. Crit Care Med. 1991;19:1352–1356. doi: 10.1097/00003246-199111000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Stacpoole PW, Wright EC, Baumgartner TG, Bersin RM, Buchalter S, Curry SH, et al. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate-Lactic Acidosis Study Group. N Engl J Med. 1992;327:1564–1569. doi: 10.1056/NEJM199211263272204. [DOI] [PubMed] [Google Scholar]
- 15.Flaveny CA, Griffett K, El-Gendy B, Kazantzis M, Sengupta M, Amelio AL, et al. Broad anti-tumor activity of a small molecule that selectively targets the Warburg effect and lipogenesis. Cancer Cell. 2015;28:42–56. doi: 10.1016/j.ccell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]