Abstract
Objective
Depression and diabetes commonly co-occur, however the strength of the physiological effects of diabetes as mediating factors towards depression is uncertain.
Methods
We analyzed extensive clinical, epidemiological and laboratory data from (n=2081) Mexican Americans aged 35 to 64 years divided into three groups: Diagnosed (self-reported) diabetes (n =335), Undiagnosed diabetes (n=227) and No diabetes (n=1519) who did not meet criteria for diabetes. Undiagnosed diabetes participants denied being diagnosed with diabetes, but on testing met the 2010 American Diabetes Association and World Health Organization definitions of diabetes. Depression was measured using the Center for Epidemiological Studies-Depression (CES-D) scale. Weighted data were analyzed using dimensional and categorical outcomes using univariate and multivariate models.
Results
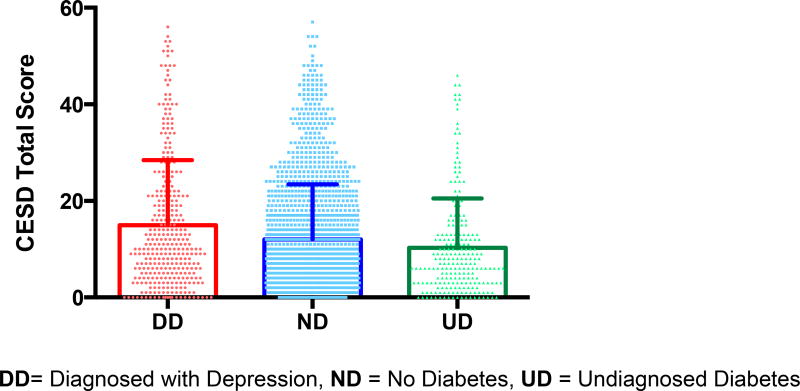
The Diagnosed diabetes group had significantly higher CES-D scores than both the No diabetes and Undiagnosed diabetes (p≤ 0.001) groups, whereas the No diabetes and Undiagnosed diabetes groups did not significantly differ from each other. The Diagnosed diabetes subjects were more likely to meet the CES-D cut-off score for depression compared to both the No diabetes and Undiagnosed diabetes groups (p = 0.001) respectively. The Undiagnosed diabetes group was also less likely to meet the cut-off score for depression than the No diabetes group (p =0.003). Our main findings remained significant in models that controlled for socio-demographic and clinical confounders.
Conclusions
Meeting clinical criteria for diabetes was not sufficient for increased depressive symptoms. Our findings suggest that the “knowing that one is ill” is associated with depressive symptoms in diabetic subjects.
Keywords: Diabetes, Depression, Mexican American, Hispanic
Introduction
The co-occurrence of diabetes and depression has been well established with the odds of depression in those with diabetes being approximately twice that of patients without diabetes (Anderson et al., 2001). The increased prevalence of depression has been reported in both type1 (Gendelman et al., 2009) and type 2 diabetes (Ali et al., 2006) and depression has been linked with poor glycemic control (Lustman et al., 2000) and diabetes complications (de Groot et al., 2001). Potential explanatory models underlying the link between depression and diabetes have included lifestyle changes and stress associated with having diabetes (Dziemidok et al., 2011) Hypothalamic Pituitary Axis (HPA) axis abnormalities (Gragnoli, 2012), and mechanisms suggesting the effect of stress on insulin resistance through inflammation, stress hormones, the rennin-angiotensin system, endothelial cells, adipocytes and the liver (Black, 2006).
Recent studies however draw into question the strength of the physiological effects of diabetes as mediating factors towards depression. A longitudinal three-year study of patients with type 2 diabetes, found the incidence of depressive symptoms was elevated only in subjects undergoing treatment for diabetes compared to subjects with impaired fasting glucose, those with normal fasting glucose, and those with untreated type 2 diabetes (Golden et al., 2008). Moreover subjects with impaired fasting glucose actually had a lower risk of depression compared to subjects with normal fasting glucose, and those with untreated type 2 diabetes had similar risk compared to those with normal fasting glucose (Golden et al., 2008). Along these lines, a recent meta-analysis noted the risk for depression was increased in individuals previously diagnosed with type 2 diabetes compared to subjects with undiagnosed diabetes and impaired glucose metabolism (Nouwen et al., 2011), furthermore the risk for depression did not differ in subjects with impaired glucose metabolism compared to those with undiagnosed diabetes (Nouwen et al., 2011). Similarly data from the National Health and Nutrition Examination Study (NHANES) revealed clinically identified type 2 diabetes was associated with an increase odds ratio of depression, but undiagnosed diabetes was not (Mezuk et al., 2013). These studies suggest that “knowing that one is ill” and being in treatment may be keys for becoming depressed in those with diabetes.
The consequences of depression and diabetes may have major public health implications for Mexican Americans. Existing studies note that diabetes is highly prevalent in Mexican American populations with approximately 25% meeting the World Health Organization’s (WHO) definition for Diabetes (Quinones et al., 2013) and depression is the most common mental illness in Mexican American subjects (Alegria et al., 2007). Our own work has found that 29% of Mexican Americans in South Texas suffer from depression (Olvera et. al., in press) and over 30% also suffer from diabetes (Fisher-Hoch et al., 2012). Herein we examine the prevalence of depression in subjects with diagnosed diabetes as well as subjects with undiagnosed diabetes and subjects without evidence of diabetes from a randomly selected population-based cohort of Mexican Americans living on the US-Mexico border.
Methods
Sample
Participants in this study were recruited between the years 2004–2013, into the Cameron County Hispanic Cohort (CCHC) (Fisher-Hoch et al., 2010). Households were randomly selected based on the 2000 census tract data in the city of Brownsville, Texas, situated on the US-Mexico border. All selected households were visited, and all occupants over the age of 18 years invited to participate. This cohort is predominantly Mexican American (>98%). Willing participants completed comprehensive questionnaires regarding basic demographic information, medical history, medication use, and social and family history as described previously (Fisher-Hoch et al., 2010). All participants provided written informed consent and this study has been approved by the Institutional Review Board of the University of Texas Health Science Center at Houston.
Measures
Based on self-reported medical history, we categorized our subjects (n=2081) into three groups: 1) “Diagnosed diabetes” (n =335) based on the subject being previously informed by a health professional that they had diabetes and meeting the 2010 American Diabetes Association (ADA) and World Health Organization (WHO) definitions of diabetes. 2) “Undiagnosed diabetes” (n=227) were those who denied being diagnosed with diabetes and were not on appropriate treatment, but who on testing met the 2010 ADA / WHO definitions of diabetes. 3) “No diabetes” (n=1519) were those who denied having received a diagnosis of diabetes, were not on appropriate treatment, and did not meet the ADA/WHO criteria for the diagnosis at the time of the visit. The 2010 ADA/ WHO definitions of diabetes is: a mean fasting blood glucose > 126 mg/dl on two consecutive visits, and/or a glycosylated hemoglobin (HbA1c) of > 6.5% (ADA, 2010)
Depression was measured using the Center for Epidemiological Studies-Depression (CES-D) a 20-item scale developed for epidemiologic studies of depressive symptoms in the general population (Radloff, 1977). Consistent with prior studies (Zich et al., 1990), we classified individuals as non-depressed if their CES-D score was < 16, and depressed if their score was ≥ 16.
Anthropometric measures were taken as described previously (Fisher-Hoch et al., 2010). Blood specimens were taken and aliquots immediately stored at −70°C for a range of clinical and experimental assays. Blood glucose measurement was performed on site, HbA1c was measured by High-performance liquid chromatography and stored specimens were sent in batches to a CLIA (Clinical Laboratory Improvement Amendments) approved clinical laboratory for clinical chemistries.
Statistical Analysis
From the original cohort of 2583 subjects, 2081 had complete data and were included in this study. These 2,081 subjects did not differ from the entire cohort in terms of age and gender status. We report results at the participant level. Our sample is 67% female therefore we incorporated sampling weights into our analysis as fully described previously to enhance generalizability (Fisher-Hoch et al., 2010). In the survey data analysis, taking into consideration of the complex sampling design, we also accounted for the potential clustering effect among participants from the same household. All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, North Carolina) and Stata 10 SE (StataCorp LP, College Station, Texas). For descriptive purposes, categorical variables for demographic and clinical characteristics were summarized in unweighted frequencies and weighted percentages. The Rao-Scott design-adjusted chi-square test was used to test for equality of proportions across the diabetes status groups. Continuous variables for demographic and clinical characteristics were summarized using weighted means and their standard errors. We assessed the overall effect of diabetes status on depression score in bivariate regression using design-based Wald F-tests. Post-Hoc pairwise comparisons of the means were assessed for significance using a Tukey-Kramer adjustment to correct for the multiple comparisons. To assess independent effects of the multiple sociodemographic factors on the CES-D score, a multivariable weighted linear regression model for depression was performed with Wald’s F tests to assess interactions. Variance inflation factor (VIF) indicated that there was not problematic multicollinearity among the independent variables in the regression model (VIF<1.5). Post-Hoc analyses using t-tests were performed comparing depression scores within subjects diagnosed with diabetes divided into those with and without reported medical complications. A Post-Hoc ANOVA was used to compare depression scores between the three groups after removing subjects with skin ulcers.
Results
We found the Diagnosed diabetes participants had significantly higher depression scores than both the Undiagnosed diabetes and No diabetes groups (p≤ 0.001) and the Undiagnosed diabetes and No diabetes groups did not significantly differ from each other on CES-D scores (see Table1, Figure 1). The Diagnosed diabetes group was significantly older than the Undiagnosed diabetes and No diabetes groups (p< 0.0001) and both the Diagnosed and Undiagnosed diabetes groups had significantly higher BMI’s than the No diabetes group (p<0.001) (see Table 1). Repeating the analyses including age, gender and BMI in the model, the difference between groups on CES-D depression scores remained significant (p< 0.001) with the Diagnosed diabetes subjects having significantly higher scores than the both the Undiagnosed diabetes and No diabetes groups respectively on pairwise comparisons (p < 0.001), with no significant difference between the Undiagnosed diabetes and No diabetes subjects. This model revealed a significant main effect for gender (p< 0.001) with females having significantly higher depression scores than males (p < 0.001) across all groups, without an interaction effect for gender.
Table 1.
Demographic and Clinical Variables: Weighted Means and Percentages
| Diagnosed with Diabetes (DD) (n=335) |
Undiagnosed with Diabetes (UD) (n=227) |
No Diabetes (ND) (n=1519) |
p | |
|---|---|---|---|---|
| m (SE) | m (SE) | m (SE) | ||
| Age | 58.00 (1.7)* | 45.93 (1.9)† | 43.11 (0.9)† | <0.0001 |
| BMI | 33.28 (0.7)* | 33.18 (0.7)* | 30.04 (0.3)† | <0.0001 |
| CESD score | 15.65 (0.9)* | 9.73 (1.0)† | 10.86 (0.4)† | <0.0001 |
| HbA1c | 7.33 (0.2)* | 7.40 (0.2)* | 4.78 (0.0)† | <0.0001 |
| HbA1c mmol/mol (IFCC units) | 57 | 57 | 29 | |
| FBG mg/dl | 171.34 (5.1)* | 134.07 (6.8)† | 96.11 (0.4)‡ | <0.0001 |
| n (%) | n (%) | n (%) | p | |
| Depression (%) | 123 (41.3%)* | 46 (17.2%)† | 452 (25.8%)‡ | <0.0001 |
| Female (%) | 228 (62.7%) | 145 (51.4%) | 1013 (55.9%) | 0.19 |
| Insured (%) | 134 (46.0%)* | 63 (31.3%)† | 401 (33.1%)† | 0.03 |
| Married (%) | 206 (62.3%) | 147 (66.9%) | 975 (63.6%) | 0.77 |
| High school education | 230 (66.1)* | 133 (49.1)† | 763 (44.1)† | <0.0001 |
| Employed Full Time | 69 (18.3)* | 70 (32.5)† | 535 (36.9)† | |
| Employed Part Time | 51 (16.7)* | 50 (22.5)† | 270 (16.4)* | <0.0001 |
| Unemployed | 215 (65.0)* | 107 (45.0)† | 714 (46.7)† | |
| Cardiovascular disease, High lipids or Cancer | 298 (91.2)* | 132 (55.9)† | 849 (58.0)† | <0.0001 |
Different superscripts *, †, ‡ denote significantly different pairwise comparisons p < 0.05
Frequencies are unewighted. Percentages use weighted data. Means and standard errors (SE) are weighted.
Abbreviations: SE = standard error; n = frequency IFCC = International Federation of Clinical Chemistry
Figure 1. CES-D Scores by Diagnostic Groups.
DD= Diagnosed with Depression, ND = No Diabetes, UD = Undiagnosed Diabetes
Using the CES-D established cut-off score of ≥ 16 as suggestive of depression 41% of Diagnosed diabetes subjects qualified as depressed whereas 26% of the No diabetes subjects and only 17% of the Undiagnosed diabetes subjects were depressed (Chi square = 19.57, df 2, p< 0.001). The increased percentage of Diagnosed diabetes subjects meeting the cutoff for depression was significant when compared to the Undiagnosed diabetes subjects (Chi square = 21.03, df1, p< 0.001) and the No diabetes group (Chi square = 10.52, df1, p = 0.001) respectively. In addition there was a significant difference between the No diabetes and Undiagnosed diabetes groups (Chi square = 4.65, df1, p =0.003).
On laboratory measures, the Diagnosed diabetes and Undiagnosed diabetes subjects had significantly higher HbA1c levels than the No diabetes group (p< 0.001) respectively, however the Diagnosed diabetes and Undiagnosed diabetes subjects did not differ from each other in terms of HbA1c. The Diagnosed diabetes subjects did have significantly higher fasting blood glucose (FBG) levels than the Undiagnosed diabetes and No diabetes participants (p< 0.001) respectively, and Undiagnosed diabetes subjects had significantly higher mean FBG levels than the No diabetes group (p< 0.001). As anticipated HbA1c and FBG were highly correlated (r= 0.66, p< 0.0001) and since these variables are used to define the presence of diabetes, we did not attempt to covary for their effects on depression. Within the total sample only FBG (not HbA1c) was modestly correlated (r= 0.074, p=0.001) with depression scores however within each group (Diagnosed Diabetes, Undiagnosed diabetes and No diabetes) neither HbA1c nor FBG were significantly correlated with depression scores.
Examining socio-demographic variables, revealed the Diagnosed diabetes subjects were significantly more likely to have insurance than the Undiagnosed diabetes group and the No diabetes group but the Undiagnosed diabetes and No diabetes groups did not differ from each other. We also found that although the Diagnosed diabetes subjects were more likely to have finished High School they also had the highest levels of unemployment compared to the other groups (Table 1).
We then explored the effects of socio-demographic variables such as gender, marital status, insurance status, education and employment in both linear (Table 2) and logistic (Table 3) regression models to examine depression scores as dimensional and categorical outcomes respectively. In the linear model the socio-demographic variable regression estimates were in the anticipated direction as female gender, and lacking a High School diploma were predictive of higher depression scores whereas being married and full time employment were significant for predicting lower depression scores. Notably, even with these socio-demographic variables in the model the Diagnosed diabetes group still was significantly higher on CES-D depression scores compared to the No diabetes subjects whereas the Undiagnosed Diabetes group and the No diabetes group did not significantly differ from each other (Table 2). Likewise, using the CES-D cutoff score in a logistic regression model, the Diagnosed diabetes group had a significantly higher Odds ratio for depression compared to the No diabetes group, whereas being Undiagnosed for diabetes was protective for depression even with other variables in the model (see Table 3). Similar to the linear model, being female and lacking a High school degree increased the risk for depression and being married and having full time employment were protective (See Table 3).
Table 2.
Linear Regression Model for CES-D Depression Score Controlling for Socio-demographic Variables*
| Estimated Regression Coefficients | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | Estimate | Standard Error |
t | p| |
| Intercept | 11.91 | 1.38 | 8.65 | <0.0001 |
|
| ||||
| Diagnosed Diabetes | 4.15 | 1.10 | 3.77 | 0.0002 |
|
|
||||
| Undiagnosed Diabetes | −0.95 | 1.04 | −0.92 | 0.3569 |
|
|
||||
| No Diabetes | 0.00 | 0.00 | ||
|
| ||||
| Female | 3.50 | 0.74 | 4.75 | <.0001 |
|
|
||||
| Male | 0.00 | 0.00 | ||
|
| ||||
| Married | −2.76 | 0.69 | −4.00 | <.0001 |
|
|
||||
| Not married | 0.00 | 0.00 | ||
|
| ||||
| Insured | −1.19 | 0.74 | −1.61 | 0.11 |
|
|
||||
| Uninsured | 0.00 | 0.00 | ||
|
| ||||
| AGE | −0.02 | 0.02 | −0.65 | 0.52 |
|
| ||||
| Full time employed | −2.02 | 0.80 | −2.54 | 0.01 |
|
|
||||
| Part time employed | −1.09 | 0.94 | −1.16 | 0.25 |
|
|
||||
| Not employed | 0.00 | 0.00 | ||
|
| ||||
| No high school | 1.61 | 0.71 | 2.28 | 0.02 |
|
|
||||
| High school | 0.00 | 0.00 | ||
all listed variables included in the model
Table 3.
Logistic Regression Model for Categorical (CESD ≥ 16) Depression by Socio-demographic Variables*
| Odds Ratio Estimates | ||
|---|---|---|
| Effect | OR | 95% CI |
| Diagnosed diabetes vs No diabetes | 1.79 | 1.16–2.78 |
| Undiagnosed diabetes vs No diabetes | 0.59 | 0.37–0.94 |
| Female vs Male | 2.13 | 1.52–2.99 |
| Married vs not married | 0.62 | 0.47–0.83 |
| Insured vs uninsured | 0.86 | 0.61–1.21 |
| AGE | 1.00 | 0.99–1.01 |
| No high school ed. vs High school ed. | 1.38 | 1.03–1.84 |
| Employed Full time vs (Not employed) | 0.58 | 0.41–0.82 |
| Employed Part time vs (Not employed | 0.85 | 0.58–1.26 |
all listed variables included in the model
When we included the presence of additional medical conditions (cardiovascular disease, high blood pressure, high lipids, and cancer) in a logistic regression model with other significant socio-demographic variables, our main findings remained unchanged as the Diagnosed diabetes group remained at higher risk of depression and the Undiagnosed diabetes subjects were at lower risk compared to the No Diabetes group (Table 4). In a Post-Hoc analysis we attempted to examine potential diabetes related medical complications within the Diagnosed diabetes group (the other groups denied such complications). Within the Diagnosed diabetes group n = 37 (11%) reported retinopathy, n = 19 (6%) reported ketoacidosis, n = 4 (1%) reported needing dialyses and 24 (7%) reported having skin ulcers. Within this group only the presence of a skin ulcer was predictive of increased depression scores, as those with ulcers had a mean CES-D of 22.21, (SE= 3.68) compared to those without = 14.44 (SE=0.73), p =0.003. Removing the 24 subjects with skin ulcers did not alter our main findings as the Diagnosed diabetes group still had significantly higher CES-D scores compared to the Undiagnosed diabetes (p< 0.001) and No diabetes (p=0.002) subjects. Within the Diagnosed diabetes group we had data on duration of diabetes in a subset (n =164) with a mean duration of 10.82 years (SE = 0.63). We did not find a significant difference in the mean duration of diabetes in depressed 10.00 years (SE=1.06) compared to non-depressed 11.29 years (SE =0.79) (p =0.33) subjects. Along these lines there was not a significant correlation between depression scores and illness duration (r = −.09, p =0.27).
Table 4.
Logistic Regression Model for Categorical (CESD ≥ 16) by Sociodemographic Sociodemographic Variables and Medical Illness
| Odds Ratio Estimates | ||
|---|---|---|
| Effect | OR | 95% CI |
| Diagnosed diabetes vs. No diabetes | 1.94 | 1.26 – 3.00 |
| Undiagnosed diabetes vs. No diabetes | 0.62 | 0.38 – 0.99 |
| Female vs. Male | 2.52 | 1.85 – 3.44 |
| Married vs. Not married | 0.63 | 0.48 – 0.84 |
| Cardiovascular disease, High lipids or Cancer | 1.14 | 0.82 –1.56 |
all listed variables included in the model
Discussion
Our main findings were that members of our cohort with Diagnosed diabetes had higher depression scores and a higher prevalence of depression compared to Undiagnosed diabetes and those with No diabetes. What is noteworthy is that participants with Undiagnosed diabetes despite having obesity, and elevated HbA1c and FBG, reported the lowest depressive symptoms and using dichotomized depression cutoffs, even had a lower odds ratio for depression relative to those in the No diabetes group. As in other studies (Egede and Zheng, 2003, Fisher et al., 2001) we found significant effects for potential socio-demographic confounders such as female gender, low education and unemployment for depression. However even when we included these variables in our models, the main effect of diabetes diagnostic status remained significant.
Our findings suggest that “knowing that one is ill” and being in treatment could be major contributors to depression in persons with diabetes. A systematic review of the responses to being diagnosed with diabetes revealed up to one half of newly diagnosed patients reporting negative emotions, however there was great variability in the emotional cognitive and behavioral responses (Thoolen et al., 2006). Many newly diagnosed patients initially downplay the seriousness of their illness (Thoolen et al., 2006) and full understanding of the implications of illness may take years (Lawson et al., 2008). Individual factors that influence the emotional response of diabetic patients to receiving the diagnosis include personality traits (Lyness et al., 1998), perceptions of illness, coping mechanisms (Bazzazian and Besharat, 2012) (Duangdao and Roesch, 2008) and severity of symptoms (Thoolen et al., 2006). An example of the complexity of this issues is the personality trait of “neuroticism” that is defined by sub-domains of worry and self-consciousness(Lane et al., 2000). In some instances neuroticism may be protective in diabetes; providing the vigilance needed for good glycemic control (Lane et al., 2000). However subjects with high neuroticism may also be at greater risk for depression (Fanous et al., 2002) especially when exposed to increased illness burden (Lyness et al., 1998). Furthermore, the “Burden of Illness” i.e. worries about complications has been associated with depression in patients with diabetes (Karlson and Agardh, 1997) suggesting there may be a tipping point where the propensity to worry combined with diabetes becomes deleterious. These concepts may account for our findings where within those diagnosed with diabetes, the presence of ulcerations, an obvious physical symptom, was significantly associated with increased CES-D scores. This suggests that there may be a certain threshold (i.e. a notable clinical manifestation of illness) that cannot be readily ignored that leads to the emergence of mood symptoms. Once the threshold is reached then a vicious cycle may occur as depression can hamper self-care and the ability to follow healthy diet and exercise (Katon et al., 2010b). In Mexican Americans with diabetes, this synergy between depression and diabetes has been documented where the presence of depression and diabetes predicted earlier mortality, and multiple complications that affected daily living (Black et al., 2003).
While there are numerous strengths of our population-based randomly recruited sample of Mexican Americans living on the US-Mexico border, there are several limitations to keep in mind when evaluating our results. First, this was a cross sectional study and therefore cannot establish causality as we did not prospectively follow them in time after receiving their diabetes diagnosis. It is possible that those with depression were more likely to receive the diagnosis of diabetes as much as it is possible that having the diagnosis of diabetes increased the risk for depression. Only a longitudinal study could disentangle these two possibilities. Second, this cohort also consisted primarily of women, was of lower SES and the majority were Mexican Americans who preferred to respond in Spanish (70%) which raises issues of acculturation, and gender that were not the primary focus of this study. Third, although the CES-D is a well-accepted measure of depressive symptoms in a population (Lewinsohn et al., 1997, Radloff, 1977), it does not follow strict DSM criteria, cannot establish chronicity and number of episodes and does not account for confounding or co-morbid or pre-existing psychiatric conditions which may play a role in depression in diabetic patients (Bot et al., 2010). Despite these limitations the breadth and sample size of this study allow for exploration based diabetic status, knowledge of illness and multiple established risk factors. Furthermore even though our sample was Mexican American our findings are identical to a large (n> 5,000) longitudinal study (Golden et al., 2008) a meta-analysis of thirteen studies comprising n= 1,483 cases (Nouwen et al., 2011) and to a nationally representative cross sectional survey (NHANES) n = 3,183 (Mezuk et al., 2013) confirming findings from samples of different racial and ethnic make-up.
Given the negative impact from having both depression and diabetes there has been added attention to addressing the emotional response to living with this serious chronic illness as part of the overall treatment. However, even though psychosocial support is recommended as a standard of care by the American Diabetes Association (American Diabetes, 2014), it is only as a category “C” as the findings are considered relatively weak with conflicting empirical evidence. Prior reviews have noted benefits from psychological and pharmacological interventions in terms of depressive symptoms but there have been mixed results for glycemic control (Baumeister et al., 2012, 2014, Markowitz et al., 2011). A collaborative care model that included pharmacotherapy, individualized goals, medication adherence monitoring, motivational coaching and self-care guide resulted in improvement across multiple domains including depression scores, glycemic control, and reports of quality of life and satisfaction compared to non-intervention controls (Katon et al., 2010a). The collaborative care intervention models’ benefits for depressive symptoms and adherence have been replicated, however, the beneficial effects of this intervention on glycemic control have not been consistent (Huang et al., 2013).
In regards to Mexican American populations, numerous individual and cultural factors have been found to impact the management of diabetes (Brown and Hanis, 2014). For example, even though lifestyle changes such a healthy diet and increased physical activity are the accepted interventions for controlling Type 2 diabetes (Tuomilehto et al., 2001), these dietary and behavioral changes, if perceived as “restrictions”, have actually been associated with increased depression in subjects with diabetes (Karlson and Agardh, 1997). Focus groups with Mexican Americans with diabetes found they did not want to participate in weight loss focused outcomes in particular those that with an emphasis on “diet” but were highly motivated by a concern for the welfare of their children and other family members (Brown and Hanis, 2014). Other issues that may be particularly salient to Mexican Americans include beliefs that being heavyset represents health (Diaz et al., 2007, Stern et al., 1982), food as a representation of love (Allan, 1998) or food security as a symbol of socio-economic status (Kumanyika, 2008) may magnify the negative perception to dietary restrictions. However culturally sensitive interventions that include family involvement and that incorporate cultural foods have been successful in better glycemic control in Mexican-Americans from the Texas-Mexico border (Brown et al., 2002).
In conclusion our study disputes the notion that Major Depressive Disorder (MDD) results directly from diabetes in favor of a multidimensional approach including consideration of biological and psychosocial factors (Talbot and Nouwen, 2000) that appears to accompany being diagnosed with diabetes. Hispanics now comprise the largest ethnic-minority group residing in the United States accounting for 15% of the population; and Mexican Americans are the single largest Hispanic subgroup and number over 46 million people (Ennis, 2011). The consequences of depression and diabetes in this population have major public health implications with the need for individualized culturally sensitive personalized treatment that includes both medical and psycho-social considerations (Dziemidok et al., 2011) (Brown et al., 2002).
Supplementary Material
Acknowledgments
We thank our support team for cohort recruitment database management and administrative support. We thank Valley Baptist Medical Center, Brownsville for providing us space for our Center for Clinical and Translational Science Clinical Research Unit. We finally thank the community of Brownsville and the participants who so willingly participated in this study in their city.
Source of funding: This work was supported by MD000170 P20 funded from the National Center on Minority Health and Health disparities (NCMHD), and the Centers for Clinical and Translational Science Award UL1 TR000371 from the National Center for Research Resources (NCRR).
Glossary
- ADA
American Diabetes Association
- ANOVA
Analysis of Variance
- BMI
Body Mass Index
- CCHC
Cameron County Hispanic Cohort
- CES-D
Center for Epidemiological Studies -Depression
- CLIA
Clinical Laboratory Improvement Amendments
- DD
Diagnosed with Depression
- FBG
fasting blood glucose
- HbA1C
Glycated Hemoglobin
- HPA
Hypothalamic Pituitary Axis
- IFCC
International Federation of Clinical Chemistry
- ND
No Diabetes
- SE
standard error
- SES
Socioeconomic Status
- UD
Undiagnosed Diabetes
- WHO
World Health Organization
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interests to disclose.
References
- ADA. American Diabetes Association. Standards of medical care in diabetes — 2010. Diabetes Care. 2010;33:S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegria M, Mulvaney-Day N, Torres M, Polo A, Cao Z, Canino G. Prevalence of psychiatric disorders across Latino subgroups in the United States. American Journal of Public Health. 2007;97:68–75. doi: 10.2105/AJPH.2006.087205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–73. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- Allan JD. Explanatory models of overweight among African American, Euro-American, and Mexican American women. West J Nurs Res. 1998;20:45–66. doi: 10.1177/019394599802000104. [DOI] [PubMed] [Google Scholar]
- American Diabetes, A. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst Rev. 2012;12:CD008381. doi: 10.1002/14651858.CD008381.pub2. [DOI] [PubMed] [Google Scholar]
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus: an abridged Cochrane review. Diabet Med. 2014;31:773–86. doi: 10.1111/dme.12452. [DOI] [PubMed] [Google Scholar]
- Bazzazian S, Besharat MA. An explanatory model of adjustment to type I diabetes based on attachment, coping, and self-regulation theories. Psychol Health Med. 2012;17:47–58. doi: 10.1080/13548506.2011.575168. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses. 2006;67:879–91. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26:2822–8. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- Bot M, Pouwer F, Ormel J, Slaets JP, de Jonge P. Predictors of incident major depression in diabetic outpatients with subthreshold depression. Diabet Med. 2010;27:1295–301. doi: 10.1111/j.1464-5491.2010.03119.x. [DOI] [PubMed] [Google Scholar]
- Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self-management education for Mexican Americans: the Starr County border health initiative. Diabetes Care. 2002;25:259–68. doi: 10.2337/diacare.25.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Hanis CL. Lessons Learned from 20 Years of Diabetes Self-Management Research With Mexican Americans in Starr County, Texas. Diabetes Educ. 2014;40:476–487. doi: 10.1177/0145721714531336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- Diaz VA, Mainous AG, 3rd, Pope C. Cultural conflicts in the weight loss experience of overweight Latinos. Int J Obes (Lond) 2007;31:328–33. doi: 10.1038/sj.ijo.0803387. [DOI] [PubMed] [Google Scholar]
- Duangdao KM, Roesch SC. Coping with diabetes in adulthood: a meta-analysis. J Behav Med. 2008;31:291–300. doi: 10.1007/s10865-008-9155-6. [DOI] [PubMed] [Google Scholar]
- Dziemidok P, Makara-Studzinska M, Jarosz MJ. Diabetes and depression: a combination of civilization and life-style diseases is more than simple problem adding - literature review. Ann Agric Environ Med. 2011;18:318–22. [PubMed] [Google Scholar]
- Egede LE, Zheng D. Independent factors associated with major depressive disorder in a national sample of individuals with diabetes. Diabetes Care. 2003;26:104–11. doi: 10.2337/diacare.26.1.104. [DOI] [PubMed] [Google Scholar]
- Ennis SR, Rios-Vargas M, Albert NG. The Hispanic Population: 2010. 2010 Census Briefs, United States Census Bureau. 2011:1–3. [Google Scholar]
- Fanous A, Gardner CO, Prescott CA, Cancro R, Kendler KS. Neuroticism, major depression and gender: a population-based twin study. Psychol Med. 2002;32:719–28. doi: 10.1017/s003329170200541x. [DOI] [PubMed] [Google Scholar]
- Fisher-Hoch SP, Rentfro AR, Salinas JJ, Perez A, Brown HS, Reininger BM, Restrepo BI, Wilson JG, Hossain MM, Rahbar MH, Hanis CM, McCormick JB. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004–2007. Prev Chronic Dis. 2010;7:A53. [PMC free article] [PubMed] [Google Scholar]
- Fisher-Hoch SP, Vatcheva KP, Laing ST, Hossain MM, Rahbar MH, Hanis CL, Brown HS, 3rd, Rentfro AR, Reininger BM, McCormick JB. Missed opportunities for diagnosis and treatment of diabetes, hypertension, and hypercholesterolemia in a mexican american population, cameron county Hispanic cohort, 2003–2008. Prev Chronic Dis. 2012;9:E135. doi: 10.5888/pcd9.110298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Chesla CA, Mullan JT, Skaff MM, Kanter RA. Contributors to depression in Latino and European-American patients with type 2 diabetes. Diabetes Care. 2001;24:1751–7. doi: 10.2337/diacare.24.10.1751. [DOI] [PubMed] [Google Scholar]
- Gendelman N, Snell-Bergeon JK, McFann K, Kinney G, Paul Wadwa R, Bishop F, Rewers M, Maahs DM. Prevalence and correlates of depression in individuals with and without type 1 diabetes. Diabetes Care. 2009;32:575–9. doi: 10.2337/dc08-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, Lee HB, Lyketsos C. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751–9. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gragnoli C. Depression and type 2 diabetes: Cortisol pathway implication and investigational needs. Journal of Cellular Physiology. 2012;227:2318–2322. doi: 10.1002/jcp.23012. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wei X, Wu T, Chen R, Guo A. Collaborative care for patients with depression and diabetes mellitus: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:260. doi: 10.1186/1471-244X-13-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson B, Agardh CD. Burden of illness, metabolic control, and complications in relation to depressive symptoms in IDDM patients. Diabet Med. 1997;14:1066–72. doi: 10.1002/(SICI)1096-9136(199712)14:12<1066::AID-DIA462>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010a;363:2611–20. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Russo JE, Heckbert SR, Lin EH, Ciechanowski P, Ludman E, Young B, Von Korff M. The relationship between changes in depression symptoms and changes in health risk behaviors in patients with diabetes. Int J Geriatr Psychiatry. 2010b;25:466–75. doi: 10.1002/gps.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanyika SK. Environmental influences on childhood obesity: ethnic and cultural influences in context. Physiol Behav. 2008;94:61–70. doi: 10.1016/j.physbeh.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Lane JD, McCaskill CC, Williams PG, Parekh PI, Feinglos MN, Surwit RS. Personality correlates of glycemic control in type 2 diabetes. Diabetes Care. 2000;23:1321–5. doi: 10.2337/diacare.23.9.1321. [DOI] [PubMed] [Google Scholar]
- Lawson VL, Bundy C, Harvey JN. The development of personal models of diabetes in the first 2 years after diagnosis: a prospective longitudinal study. Diabet Med. 2008;25:482–90. doi: 10.1111/j.1464-5491.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–87. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- Lyness JM, Duberstein PR, King DA, Cox C, Caine ED. Medical illness burden, trait neuroticism, and depression in older primary care patients. Am J Psychiatry. 1998;155:969–71. doi: 10.1176/ajp.155.7.969. [DOI] [PubMed] [Google Scholar]
- Markowitz SM, Gonzalez JS, Wilkinson JL, Safren SA. A review of treating depression in diabetes: emerging findings. Psychosomatics. 2011;52:1–18. doi: 10.1016/j.psym.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk B, Johnson-Lawrence V, Lee H, Rafferty JA, Abdou CM, Uzogara EE, Jackson JS. Is ignorance bliss? Depression, antidepressants, and the diagnosis of prediabetes and type 2 diabetes. Health Psychol. 2013;32:254–63. doi: 10.1037/a0029014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwen A, Nefs G, Caramlau I, Connock M, Winkley K, Lloyd CE, Peyrot M, Pouwer F European Depression in Diabetes Research, C. Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium. Diabetes Care. 2011;34:752–62. doi: 10.2337/dc10-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones AR, Liang J, Ye W. Differences in diabetes mellitus onset for older Black, White, and Mexican Americans. Ethn Dis. 2013;23:310–5. [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385. [Google Scholar]
- Stern MP, Pugh JA, Gaskill SP, Hazuda HP. Knowledge, attitudes, and behavior related to obesity and dieting in Mexican Americans and Anglos: the San Antonio Heart Study. Am J Epidemiol. 1982;115:917–28. doi: 10.1093/oxfordjournals.aje.a113379. [DOI] [PubMed] [Google Scholar]
- Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Care. 2000;23:1556–62. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- Thoolen BJ, de Ridder DT, Bensing JM, Gorter KJ, Rutten GE. Psychological outcomes of patients with screen-detected type 2 diabetes: the influence of time since diagnosis and treatment intensity. Diabetes Care. 2006;29:2257–62. doi: 10.2337/dc06-0617. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Zich JM, Attkisson CC, Greenfield TK. Screening for depression in primary care clinics: the CES-D and the BDI. Int J Psychiatry Med. 1990;20:259–77. doi: 10.2190/LYKR-7VHP-YJEM-MKM2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.