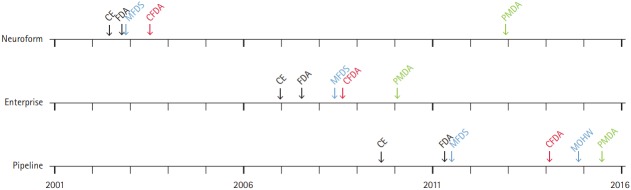
Figure 5.
Timeline of regulatory process for representative medical devices from Neuroform, Enterprise, and Pipeline in Korea (blue), China (red), and Japan (green). During the period between Neuroform and Pipeline approvals, the processing time for permission increased in Korea. Note the two-step approval process in Korea for the Pipeline as a new device: first by the Ministry of Food and Drug Safety (MFDS) and next by the Ministry of Health and Welfare (MOHW). The reason of import delay for Neuroform in Japan was that Neuroform EZ, the fourth generation device, was first introduced in Japan. Such time delay may also be due to cost differences among countries; Neuroform and Enterprise cost approximately 1,400 USD in Korea, 4,000 USD in China, and 4,200 USD in Japan, whereas the Pipeline costs 9,000 USD in Korea, 13,000 USD in China, and 13,900 USD in Japan. CE, Conformité Européenne (European Conformity); FDA, U.S. Food and Drug Administration; CFDA, China Food and Drug Administration; PMDA, Pharmaceuticals and Medical Devices Agency.