Figure 2.
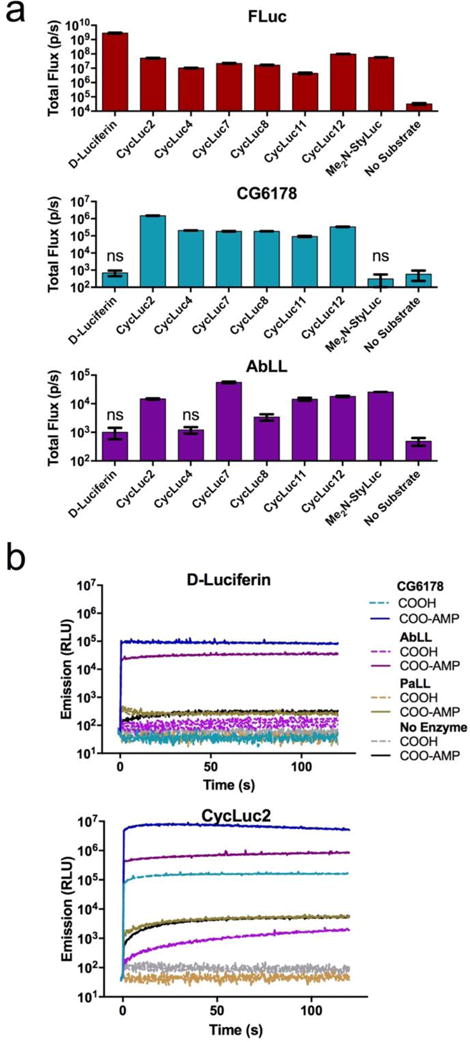
ACSL luciferase activity. (a) Identification of emissive substrates for ACSLs. Each enzyme (20 nM) was treated with the indicated luciferin analog (250 μM). Significant flux over no-substrate control was observed (p < 0.05) unless marked “ns”. (b) Burst kinetics profiles of each ACSL with D-luciferin, CycLuc2, and their pre-activated adenylates. Purified enzyme (100 nM) was rapidly injected into substrate (100 μM). Light emission was recorded every 0.5 s for 1 s pre-injection and 120 s post-injection. Background luminescent signal in the absence of enzyme is shown for reference (in gray). All assays were performed in triplicate, are represented as the mean ± SEM, and are presented on the same log scale.
