Abstract
Decline in associative memory abilities is a common cognitive complaint among older adults and is detectable in both normal aging and in prodromal Alzheimer’s disease (AD). Subjective memory (SM) complaints may serve as an earlier marker of these mnemonic changes; however, previous research examining the predictive utility of subjective memory to observed memory performance yielded inconsistent results. This inconsistency is likely due to other sources of variance that occur with memory decline such as mood/depression issues, presence of Apolipoprotein E (APOE ε4) genotype, or beta-amyloid deposition. Here we examine the relationship between SM and associative memory ability in the context of factors that increase susceptibility to AD in 195 healthy adults (79 men) aged 20–94 years. Participants completed a subjective memory questionnaire, a mood/depression scale, two associative memory tests (a word-pair and a name-face test) and were genotyped for APOE ε4. PET-amyloid imaging data were collected for a subset of those over 50 years of age (N= 74). We found that SM predicted performance on both associative memory tests even after covarying for age, sex, mood, and APOE ε4 status. Interestingly, for the name-face associative task, increased subjective memory concerns predicted memory performance selectively in participants over the age of 60, with APOEε4 risk group showing the strongest effect. Finally, men with higher beta-amyloid deposition reported more memory complaints. Our findings suggest that SM reliably tracks memory performance, even in cognitively healthy adults, and may reflect an increased risk for Alzheimer’s disease.
Keywords: Subjective Memory, Associative Memory, Aging, Apolipoprotein E, Beta-Amyloid
Introduction
In Alzheimer’s disease (AD), neuropathological changes begin a decade or more prior to clinical expression of the disease (Jack et al., 2013), and up to 20% – 30% of older adults in studies of healthy cognitive aging may harbor preclinical AD (Pike et al., 2007; Rodrigue et al., 2012). Thus, there is a critical need to identify tools that can detect subtle, preclinical changes in cognitive function before a significant amount of neural deterioration transpires. Behaviorally, older adults with early pathological brain changes are virtually indistinguishable on standard measures of cognitive performance. A supplemental approach to assessing an individual’s decline in cognitive performance is the use of a subjective memory (SM) assessment tool, a self-evaluation measurement of memory changes. The rationale for using subjective memory survey instruments to detect changes in memory performance is grounded in the idea that individuals may be sensitive to small, initial declines in their own memory performance that are clinically meaningful. This assessment approach may be particularly useful in cross-sectional studies of non-pathological aging, where traditional neuropsychological assessment is unable to capture change in performance within the individual.
Overall, research indicates that SM complaints may be indicative of the emergence of early phases of pathological cognitive decline such as mild cognitive impairment (MCI) and AD (Clarnette et al., 2001; Jonker et al., 1996). Longitudinally, research participants who express subjective memory complaints appear to be at greater risk for future cognitive decline (Dik, et al., 2001; Glodzik-Sobanska et al., 2007) and the eventual development of AD (Geerlings et al., 1999; Schmand et al., 1996; Schmand et al., 1997; Wang et al., 2004), although some evidence from very old, healthy adults indicated no relationship between SM complaints and memory performance. (Pearman et al., 2014). A recent study that compared SM complaints across clinical diagnosis groups found that although only one-third of individuals with complaints received a classification of either MCI or AD, no participants who reported having normal memory demonstrated clinically meaningful cognitive deficits (Jacinto et al., 2014), illustrating the sensitivity of subjective memory assessment in tracking normal as well as pathological aging.
Human cognitive aging, even in an optimal presentation, is accompanied by numerous changes to cognitive function, including moderate age-related decrements in episodic memory performance (Verhaeghen, Marcoen & Goossens, 1993). Within episodic memory, associative memory is a particularly sensitive percept to both normal and pathological aging, with evidence indicating associative memory tasks are more sensitive to early cognitive changes in older adults (Naveh-Benjamin, 2000; Naveh-Benjamin et al., 2003; Sperling et al., 2014). Deficits in performance are more pronounced in associative tasks compared to item memory, such as learning face-name pairs, a cognitive domain which is often reported as the most common cognitive complaint among older adults (Fowler et al., 2002; Naveh-Benjamin et al., 2004). There have been few investigations on the link between SM complaints and associative memory specifically, with most reporting no significant association (e.g., Derouesne et al., 1999; Kahn et al., 1975; Minett et al., 2008). However, recent work (Polcher et. al., 2017) illustrated that performance on a face-name task distinguished between normal aging, those with SM complaints, and an MCI group. Thus, it is possible that associative memory processes may be especially sensitive to the subtle differences between normal aging and the early pathological changes in memory.
An important factor to consider in the evaluation of the link between subjective and objective memory is individual differences in affect. Some evidence from healthy aging studies suggests that SM complaints are not indicative of cognitive decline, per se, but may instead be tracking factors such as personality traits (Hänninen et al., 1994), anxiety and depression (Derouesne et al., 1999), or general emotional status (Smith et al., 1996) rather than objective memory performance (Pearman et al., 2014; Riedel-Heller et al., 1999). SM complaints may be most sensitive to the degree of an individual’s depression, regardless of their actual cognitive performance in the laboratory (Kahn et al., 1975; Minett, 2008). The influence of depression on cognition is particularly relevant in the context of older adults. Not only does depression itself potentially impair cognition (Golinkoff & Sweeny, 1989), but the two frequently coexist in geriatric patients (Reifler et al., 1982). Thus, it is currently unclear to what extent SM complaints may actually primarily reflect underlying individual differences in affect and mood rather than cognitive changes.
The presence of risk factors for AD, such as APOE genotype or beta-amyloid deposition, may also influence the association between SM and objective memory performance. Apolipoprotein (APOE) ε4 allele is a major susceptibility gene for Alzheimer’s disease with the risk of developing AD increasing and the age of onset decreasing, with each additional ε4 allele (Corder et al., 1993). SM complaints are more prevalent in APOE ε4+ carriers than in their ε4- counterparts (Small et al., 2001; Stewart et al., 2001), and APOE ε4+ participants perform significantly worse on delayed recall than non-carriers, despite no differences in measures of attention, vocabulary, or immediate recall (O’Hara et al., 1998). Therefore, individuals with increased genetic risk for AD might be able to perceive change in memory abilities before deficits are clinically discernible as well as demonstrate poorer memory overall compared to those without such risk, underscoring the importance of measuring the effect of the APOE polymorphism in normal aging studies.
Interestingly, a few studies provide evidence in support of the hypothesis that beta-amyloid accumulation may be related to SM complaints in even normal aging studies. Beta-amyloid plaque is a biomarker for Alzheimer’s disease (Hardy & Selkoe, 2002) that accumulates years before clinically detectable cognitive deficits emerge (Sperling et al., 2011). Individuals with greater amyloid burden report increased SM complaints (Amariglio et al., 2012; Perrotin et al., 2012; Mielke et al., 2012) and there is additional evidence that this relationship may be selective to APOE ε4 carriers (Mosconi et al., 2008; Rowe et al., 2010). Interestingly, discrepancies between memory performance and perceived memory ability in healthy participants and individuals with MCI may vary with amyloid extent; Individuals with MCI tended to overestimate their memory performance as amyloid accumulation increased, whereas healthy individuals were more likely to underestimate their performance (Vannini et al., 2017).
Overall, it appears that individual self-reports of memory function may be sensitive to objective memory performance in both healthy aging samples and studies of pathology. The mix of both null and positive associations reported between subjective reports and memory measurements in healthy aging studies in particular, may reflect differences in inclusion of important modifying variables such as mood or depression status, APOE genotype, and beta-amyloid burden across studies. Given the abundant work documenting memory decline across the lifespan (e.g., Craik & Jennings, 1992; Light, 1992; Nyberg, et al., 1996), many healthy adults will have experienced some change from their previous memory ability, as early as in the 30s and especially by later life, i.e., 60+ years of age (e.g., Park et al., 2002). Thus, tapping into an individual’s sense of change over time may make subjective memory assessments an informative gauge of real changes in within-person memory decline, with associative memory in particular serving as an age-sensitive domain in which to study.
The present study aimed to investigate the relationship between subjective memory assessment and laboratory measures of memory performance, while taking into consideration the above-mentioned potential sources of variance in individual differences. We focused on associative memory performance specifically, given the early age-related changes in this cognitive domain previously reported and the fact that associative memory failures are the most common age-related cognitive complaint. First, we sought to examine the relationship between SM assessment and associative memory performance. We hypothesized that individuals with the most SM complaints would perform more poorly than those with fewer complaints. Further, because within episodic memory, deficits in performance are more pronounced in associative than item memory tasks, such as learning face-name pairs (Fowler et al., 2002), we hypothesized that subjective memory would be more predictive of associative memory performance than item memory performance and the relation to individual difference variables would be more strongly related to associative memory than to item memory. Second, in investigating the link between these individual difference measures in the context of factors that increase susceptibility to AD, we hypothesized the relationship would be stronger in cognitively healthy individuals at greater genetic risk to develop AD: APOE ε4+ carriers. We also hypothesized that individuals with elevated beta-amyloid deposition would report greater subjective memory complaints.
Methods
Participants
The full sample consisted of 195 healthy volunteers (79 men, 116 women) aged 20–94 (M = 53.82, SD = 18.51 years), recruited from the Dallas-Fort Worth area using flyers and media advertisements. Participants were educated at about college level (M = 15.61, SD = 2.44) and screened against a variety of health factors including neurological and psychological disorders, head trauma, cancer, alcohol or substance abuse, and medications known to affect cognition. Participants were screened against depression using Center for Epidemiological Study Depression Scale (CESD; Radloff, 1977) and dementia via Mini Mental State Exam (MMSE; Folstein et al, 1975), with cutoff scores of > 16 and < 26 respectively. Participant demographics are presented in Table 1. This study was conducted under approval of University of Texas at Dallas and University of Texas Southwestern Medical Center review boards.
Table 1.
Sample demographics by sex and by APOE genotype
| Men | Women | Total Sample | |||
|---|---|---|---|---|---|
| APOE ε4− | APOE ε4+ | APOE ε4− | APOE ε4+ | ||
| N | 63 | 16 | 100 | 16 | 195 |
| Mean Age (SD) | 52.13 (±17.40) | 56.13 (±19.48) | 54.42 (±19.44) | 54.38 (±16.94) | 53.82 (±18.51) |
| Age Range | 20–87 | 21–78 | 20–94 | 21–86 | 20–94 |
| MMSE (SD) | 28.78 (±1.01) | 29.06 (±0.68) | 29.06 (±0.93) | 29.00 (±0.82) | 28.96 (±0.93) |
| Education (SD) | 16.13 (±2.51) | 14.00 (±2.42)* | 15.57 (±2.22) | 15.38 (±2.90) | 15.61 (±2.44) |
| CESD (SD) | 3.87 (±4.06) | 3.56 (±3.44) | 4.36 (±3.82) | 4.63 (±3.18) | 4.16 (±3.81) |
Note. MMSE = Mini Mental State Exam; CESD = Center for Epidemiological Study – Depression Scale;
Denotes p < .05 difference between lowest and highest subgroup.
Procedures
Upon enrollment into the study, the participants underwent (in order) two cognitive testing sessions of approximately 2.5-hours in length, on two separate days to prevent fatigue (Session 1 and Session 2), and provided a saliva sample for genotyping of Apolipoprotein E (APOE) during Session 2, magnetic resonance imaging scanning on Session 3, and, for a subsample of the participants, beta-amyloid PET imaging during Session 4. Although a comprehensive neuropsychological and cognitive test battery was administered as part of the larger study, the present study focused on the tasks described below.
Behavioral Measures
Episodic Memory Tasks
Memory for Names (MfN)
The Memory for Names subtest of the Woodcock Johnson-III Psychoeducational Battery (Woodcock et al., 2003) was administered during Session 1. MfN measures visual associative memory through the learning of twelve space creatures with unique features and novel, nonsensical names. Participants are serially presented one space creature image along with its name presented aloud by the experimenter. After each trial, participants are asked to identify (by pointing to) the creature first alone and then from amongst an array of previously learned creatures until all 12 are presented. Participants are asked to learn the name of a new creature while remembering the identity of all previously learned creatures with feedback given if an incorrect response is given. Each creature is presented six times during the learning portion of the test. After a 30–40 min (incidental) delay, the participant is then required to point to a creature from an array of several creatures previously studied when its name is presented, with three trials per array, with no feedback on correctness. Scores for this task are total correct responses during the immediate learning portion (out of 72 possible) and total correct responses during the delayed recognition portion (out of 36 possible). The two measures (immediate, delayed) were standardized into a Z-score (with mean of zero and SD of 1) with Cronbach’s α = .943.
Verbal Paired Associates (VPA)
The Verbal Paired Associates subtest of the Wechsler Memory Scale (Wechsler, 2009) was administered at the beginning of Session 1. VPA is a verbal associative learning task in which fourteen word pairs are orally presented to participants at a pace of one word per second with a one second pause between each word pair. Some of the word pairs are semantically related (street, road) while others have no semantic association (bag, truck). After each presentation, the examiner states the first word of each pair and asks the participant to recall and respond with the corresponding word, correcting participants if they are incorrect. This is repeated for four trials. After a 20–30 minute delay, participants are orally presented the first word of the word pair and asked to recall the second word of the pair with no feedback. Scores for this task are the total correct responses during the immediate learning portion (out of a possible 56) and total correct responses during delayed cued recall (out of a possible 14). The two measures (immediate and delayed) were standardized into a Z-score composite (with mean of zero and SD of 1) with Cronbach’s α = .925.
California Verbal Learning Test (CVLT)
The California Verbal Learning Test 2nd Ed. (CVLT-II; Delis et al., 2000) was administered during Session 1. Participants are orally presented a list of 16 words that can be categorized into four semantic categories: transportation, vegetables, furniture, and animals. The list is read at a pace of one word per second. Following the reading of the list, participants are asked to recall as many words as they can, in any order and this is repeated across five trials of presentation of these 16 words. This test produces several indices of performance but since our interest is in an item memory index we selected the sum of correct responses across all five trials as our measure of interest (out of a possible 80).
Self-report Inventories
Memory Function Questionnaire (MFQ)
The MFQ (Gilewski, Zelinksi, & Schaie, 1990; Revell, Caskie, Willis, & Schaie, 2001) is an index of subjective memory complaints that includes four subscales including General Frequency of Forgetting, Seriousness of Forgetting, Retrospective Functioning, and Mnemonics Usage. For the current study, we selected one major subscale, the General Frequency of Forgetting subscale due to its high face validity for episodic memory failures. We elected to use this subscale on its own due to lower intercorrelations with other subscales that were found when assessing subjective memory in a lifespan study (Gilewski 1990). Evidence suggests that the Frequency of Forgetting subscale is more sensitive to cognitive performance than other scales both cross-sectionally (Hertzog et al., 2000; Weber et al; 2012) and longitudinally (Parisi et al., 2011). This subscale requires participants to rate statements about failures of episodic memory (e.g., “How often do you have trouble remembering names or faces”) by answering questions about the frequency of memory problems for 33 different kinds of information using a 7-point Likert scale with 1 indicating “always a problem” and 7 indicating “no problem”. Item responses for the MFQ are summed together with higher scores reflecting fewer subjective memory failures.
Center for Epidemiologic Study – Depression Scale (CES - D)
This survey consists of twenty questions answered on a four-point Likert scale with a possible range of 0–80 total points, with lower total scores indicating fewer depressive symptoms. The present sample was screened for signs of clinical depression using the CES - D (Radloff, 1977) with a score of sixteen as a maximum cut–off criterion for study inclusion. At pre-screening, 33 individuals scored > 16 and did not enter the study. Although we exclude depression from the project to control for its potential confounding effects on cognitive aging, the CES - D shows a wide range of possible scores and was implemented in this study to quantify differences in affect across participants that may impact subjective estimates of memory ability and measures of cognition. CESD scores ranged from 0–16 with a mean of 4.16 ± 3.81 SD (see Table 1).
APOE Genotyping
Saliva samples were collected from participants using Oragene collection tubes (DNA Genotek, Ottawa, ON, Canada). DNA extraction and genotyping assays were conducted at the University of Texas Southwestern Medical Center Microarray Core Facility. For genotyping quality control, 10% direct repeats and DNA sequencing for verification were performed. We used both control DNA and no-template controls and ran all 5′-nuclease assays using Applied Biosystem’s 7900HT Fast Real-Time PCR System. DNA was extracted from saliva samples using the prepIT-L2P purifier reagent (DNA Genotek, Ottawa, ON, Canada) and following the procedure in the manual purification protocol handbook. DNA integrity was determined by subjecting the samples to electrophoresis with 1% agarose gels. Polymorphism for APOE (SNPs rs429358 and rs7412) was ascertained using Taqman SNP Genotyping assays (Applied Biosystems) with DNA sequencing reactions carried out using the 0.5X protocol for ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems). Participants were considered APOE ε4 carriers (APOE ε4+) if they possessed one or more ε4 alleles. There were 163 APOE ε4- and 32 APOE ε4+ individuals (see Table 1).
PET and MRI Protocols
A subsample of individuals over the age of 50 (N = 74; 31 men) underwent beta-amyloid PET imaging. These participants ranged in age from 50–94 (M = 67.68, SD = 10.59), had about college level education (M = 15.64, SD = 2.625), were not cognitively impaired (MMSE M = 28.77, SD = 0.80), nor depressed (CESD M = 3.61, SD = 3.63). Participants were injected with a 10 mCi bolus of 18F-Florbetapir. At 30 min post-injection, participants were positioned on the imaging table of a Siemens ECAT HR PET scanner. Soft Velcro straps and foam wedges were used to secure the participant’s head and the participant was positioned using laser guides. A 2 min scout was acquired to ensure the entire brain was completely in the field-of-view and there was no rotation in either plane. A 2-frame by 5-min each dynamic emission acquisition was started 50 min post-injection and immediately after an internal rod source transmission scan was acquired for 7 min. The transmission image was reconstructed using back projection and a 6mm FWHM Gaussian filter. The emission images were processed by iterative reconstruction, 4 iterations and 14 subsets with a 3mm FWHM ramp filter.
All participants underwent magnetic resonance imaging including a T1-weighted high-resolution anatomical MRI scan (160 sagittal slices, 1 x 1 x 1 mm3 voxel: 204 x 256 x 160 matrix, TR = 8.1 ms, TE = 3.7 ms, flip-angle = 12˚), acquired on a 3T Philips Acheiva scanner. Each PET scan was coregistered to the corresponding participant’s T1-weighted high-resolution anatomical MRI scan using ANTs (Avants et al., 2009). No partial volume correction was performed. FreeSurfer software pipeline (Dale et al., 1999) was used to derive regions of interest from the T1-weighted images in each participant in native space and then the coregistered PET images were used to extract uptake counts from the regions of interest. Mean cortical Standardized Uptake Value Ratio (SUVR) was computed as a continuous measure of amyloid burden by averaging uptake counts from seven cortical regions of interest and standardized to whole cerebellum uptake (similar to e.g., Mintun et al., 2006; Rodrigue et al., 2012). These seven regions (Middle and Inferior Frontal, Lateral Temporal, Lateral Parietal, Anterior and Posterior Cingulate, Precuneus) are standard to the field as they represent cortical areas known to contain beta-amyloid plaques and to have measureable uptake of the ligand.
Results
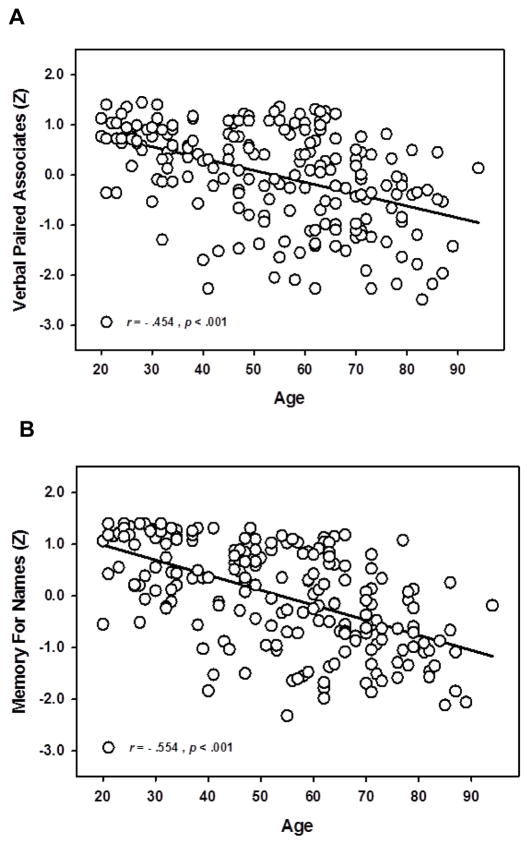
The first objective of this study was to examine the relationship between subjective memory assessment and objective memory performance on associative memory constructs. We conducted general linear models with cognitive test composite scores as continuous dependent variables and included age (continuous), sex, MFQ score (continuous), and APOE status (ε4- or ε4+), as well as their interactions as predictors. CESD was included as a covariate. Interactions that were not significant (p > .1) were removed and the model was rerun to conserve statistical power. Both associative memory constructs showed significant age-related decline across the adult lifespan as illustrated in Figure 1.
Figure 1.
Associative memory across the adult lifespan. Verbal Paired Associates (A) and Memory for Names (B) scores show significant age-related decline across the lifespan. Memory test scores are expressed in standardized units.
Subjective Memory and Associative Memory Performance: Verbal Paired Associates
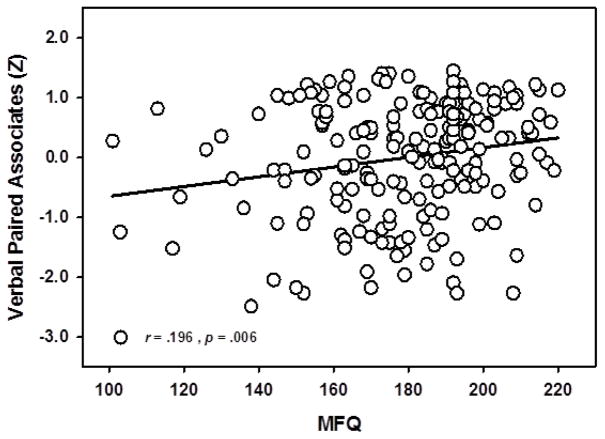
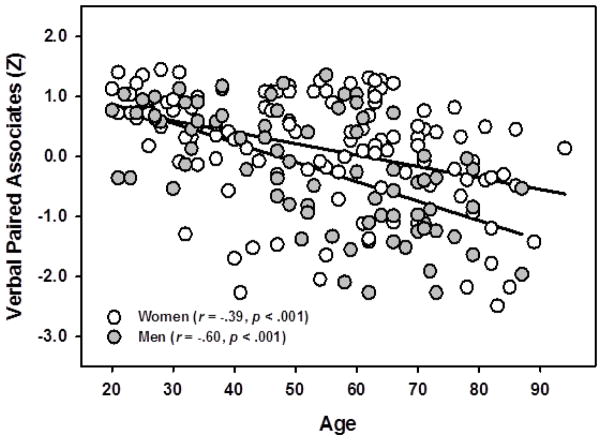
Results indicated that SM predicted associative memory performance on the VPA task [F(1,187) =6.20, p =.014], see Figure 2. Significant main effects of age on VPA [F(1,187) =51.12, p < .001] were found, with older participants showing worse memory performance than younger participants, and sex, with women remembering more word-pairs than men [F(1,187) =4.03, p =.046]. These effects were qualified by a significant sex x age interaction [F(1,187) =4.83, p =.029] where the age-related decrease in recall was significantly stronger for men (r = −.60, p < .001) than women (r = −.39, p < .001), See Figure 3. This model accounted for approximately 30% of the total variance in VPA scores (R2 = .293).
Figure 2.
Main effect of Subjective Memory on Verbal Paired Associates. Subjective memory assessment significantly predicted associative memory performance on the Verbal Paired Associates task, after controlling for age, sex, and mood/depression variables (CES - D). Lower scores on MFQ indicate more memory complaints. Memory scores expressed in standardized units.
Figure 3.
Interaction of Sex and Age on Verbal Paired Associates. Men experienced steeper decline with age on Verbal Paired Associates memory than did women. Memory scores expressed in standardized units.
Subjective Memory and Associative Memory Performance: Memory for Names
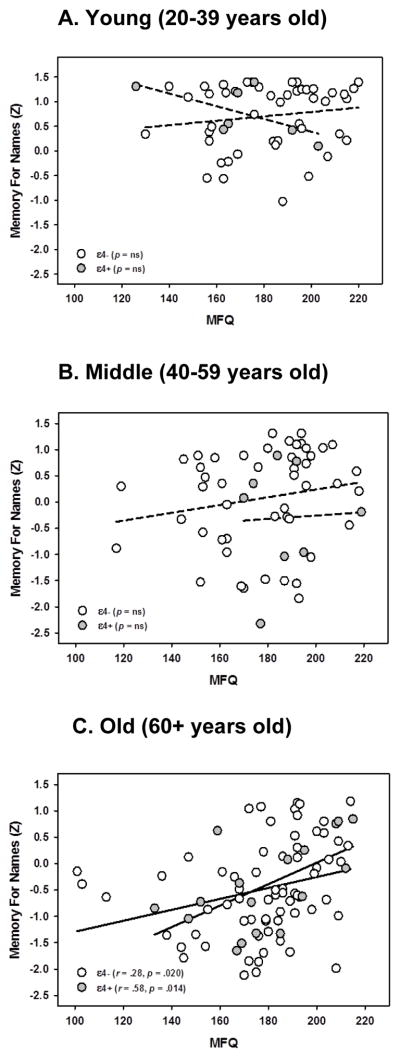
In the MfN associative memory task we found a main effect of age that indicated older participants remembered fewer of the name-face pairs than younger participants [F(1,181) = 8.16, p = .005], as well as a main effect of APOE genotype with ε4+ carriers performing significantly worse than those not carrying an ε4 allele [F(1,181) = 3.81, p = .052]. However, these main effects emerged significantly in an interaction with SM complaints, APOE x age x MFQ [F(1,181) = 4.43, p = .037]. Decomposition of the interaction revealed that older individuals in both APOE ε4 carrier and non-carrier groups were driving the interaction: there was no reliable effect in the younger (20–39) or middle-aged adults (40–59), whereas those aged 60 and up showed a significantly stronger association in the ε4+ (r = .58, p = .014) than ε4- (r = .28, p = .020) group, see Figure 4a, b, and c. The individuals with fewer subjective memory complaints showed better recognition for both groups, but the relationship was strongest in those with an increased genetic risk for AD. This model accounted for almost 40% of the total variance in MfN scores (R2 = .388).
Figure 4.
Three-way interaction between Age, Subjective Memory, and APOE carrier status on Memory for Names. For participants 60 years or older (panel c), Subjective Memory Impairment was associated with memory performance, with higher subjective memory associated with more items recognized, and lower subjective memory associated with fewer items recognized. This relationship was stronger in individuals who carried the APOE ε4+ allele than for those without an ε4 allele. There was no such significant relationship for the young (panel a) or middle-aged (panel b) individuals. Lower scores on MFQ indicate more memory complaints. Memory scores expressed in standardized units.
Subjective Memory and Item Memory Performance: California Verbal Learning Test
To test the specificity of SM complaint to associative memory, we also investigated SM effects on CVLT item memory as a “control” process. On this task, we found a main effect of SM complaints, indicating that individuals with fewer complaints recalled more items than those with more complaints, F(1,187) = 12.749, p < .001. This analysis also revealed a significant main effect of age with participants remembering fewer items as age increases, F(1,187) = 17.37, p < .001. APOE ε4+ carriers performed significantly poorer than those not carrying an ε4 allele, F(1,187) = 4.54, p = .034. A significant sex x age interaction revealed that men declined at a faster rate (r = −.562, p < .001) than women (r = −.382, p < .001). Importantly, the effect of SM on item memory did not interact with any individual difference variables, as was the case for the between SM and associative memory. This model accounted for almost 35% of the total variance in CVLT scores (R2 = .341).
Beta-Amyloid and Subjective Memory
The second goal of this study was to examine the relationship between beta-amyloid deposition and SM complaint. We conducted general linear models with MFQ scores as the continuous dependent variables and included age (continuous), sex, beta-amyloid SUVR (continuous), as well as their interactions as predictors. CESD was included as a covariate. Non-significant interactions (p > .1) were removed and the model was rerun to conserve statistical power.
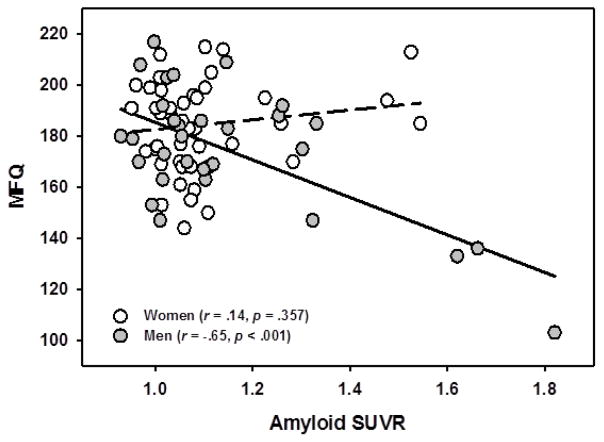
We found a main effect of sex [F(1,68) = 12.89, p = .001] which indicated men had more memory complaints than women. There was also a trending main effect of amyloid [F(1,68) = 3.36, p = .071], indicating that individuals with more amyloid deposition had more complaints about their memory. These main effects emerged significant in a sex x amyloid interaction [F(1,68) = 15.23, p < .001], driven by the fact that men with higher amyloid reported the most impairment in their memory (r = −.65, p < .001), whereas there was no significant relationship for women (r = .14, p = .357), See Figure 5. We note that these are small subgroups and caution is warranted in their interpretation. Removal of the outlying data point, however did not alter the significance. This model accounted for 34% of the total variance in subjective memory score (R2 = .340).
Figure 5.
Interaction of Sex and Amyloid in predicting Subjective Memory. Men with higher amyloid endorsed more subjective memory complaints than men without amyloid. There was no relationship for women. Lower scores on MFQ indicate more memory complaints.
Discussion
The present study investigated the relationship between self-reported impairment in memory abilities and objectively measured associative memory performance in healthy adults across the lifespan. Additionally, we investigated how this relationship is modified by mood/affect and APOE ε4 genotype, and whether extent of beta-amyloid deposition predicted degree of SM complaint. The results indicated that participants’ subjective memory assessment was a valid predictor of objective performance on both associative memory tasks examined (and in item memory), and that amyloid deposition predicted the amount of subjective memory complaints in a subsample of participants. These results suggest that individuals who self-report greater memory impairment perform more poorly on memory tasks and that this relationship is, in part, specific to adults with risk factors for AD.
We detected age and sex effects on memory performance that are in accordance with the extant literature on episodic memory across the lifespan (Park et al., 2002). Older participants performed worse on both associative memory tasks compared to younger participants. Previous research has shown that episodic memory performance declines with aging and is often one of the first domains to show clinically significant cognitive decline in preclinical AD (Grober et al., 2000; Hodges, 1998; Linn et al., 1995; Park et al., 2002; Small et al., 1997). In regard to sex differences, we observed better performance in women than men on memory, consistent with previous research (Herlitz et al., 1997; Herlitz et al., 1999), including evidence that women sometimes perform better on episodic memory tasks involving a verbal component (Bolla-Wilson & Bleecker, 1986; Geffen et al., 1990; West et al., 1992, Zelinski et al., 1993). We found this to be true on the word pair task, which only involved verbal cues and responses, as opposed to the name-face task which included predominately visual stimuli. This sex effect was exacerbated with age, where, although performance for both men and women declined across the lifespan, men showed greater age-related decrease in memory scores than women (as in Naveh-Benjamin et al., 2012). Further, we observed these significant predictive effects of subjective memory on both of our associative memory tasks even when accounting for differences in affect. This lends some support to the idea that subjective memory complaints are not entirely reflective of depressed mood states (for a broader discussion see Hertzog & Pearman, 2014).
In non-cognitively normal groups, there is evidence that associative memory tests are sensitive enough to distinguish between individuals with SM impairment, MCI, and AD (Polcher et al., 2017). Our findings lend some support to this, as we report stronger findings in individuals carrying a significant AD risk factor. Genetic risk for AD, as assessed via APOE ε4 carrier status, predicted memory performance on one associative memory task. On the MfN task, we found that greater subjective memory complaints predicted poorer recall performance in all older adults (age 60–94), although APOE ε4+ carriers showed a significantly stronger relationship between subjective and objective memory. This relationship was not present for middle-aged or younger adults. This associative memory task is arguably the more difficult task of the two measures examined and more representative of episodic memory failures that individuals might encounter in everyday life. Indeed, we previously found that individuals with genetic predisposition to lower brain-derived neurotrophic factor, performed more poorly on this task and reported greater number of memory complaints (Kennedy et al., 2015). Previous research also demonstrated a relationship between SM complaints and APOE ε4 carrier status (Laws 2002; Small et al., 2001; Stewart et al., 2001).
Further, we investigated whether amyloid deposition could predict SM complaints in older individuals. There was a significant relationship between amyloid deposition and SM complaints specifically in men, such that a higher amyloid burden was detected in the men with the most complaints about their memory. Previous research has indicated a relationship between SM complaints and amyloid, but did not report a sex specific relationship (Amariglio et al., 2012; Barnes 2006; Perrotin et al., 2012), however it is important to keep in mind that these subgroups are fairly small. Some literature only found the relationship between amyloid burden and SM complaints in individuals with APOE ε4 risk, but not for individuals without such risk, suggesting that multiple AD risk factors may interact to predict SM complaints (Mosconi et al., 2008; Rowe et al., 2010).
Importantly, the current research utilized associative memory tasks which most similarly mimic the kinds of memory failures that older adults commonly experience with aging (Naveh-Benjamin et al., 2004). Comparing SM complaints to a task with high face-validity indicates that an individual’s complaints about their memory are related to the amount of failures of their memory. By incorporating risk factors for AD that would precede changes in cognitive ability, we found that SM complaints related to associative memory performance in specific groups. Within our sample, the oldest individuals with genetic risk for AD showed the strongest relationship between their subjective and objective memory. Individuals with high amyloid had more memory complaints as well. By combining a task that is sensitive enough to discriminate between healthy and cognitively impaired individuals with risk factors for AD, the current research was able to demonstrate that SM complaints are indeed related to memory performance differences, especially within certain at-risk populations. Interestingly, although subjective memory complaints were significantly related to item memory, this relation was a straightforward one, as compared to the complex interactions seen in our associative memory findings. However, it also suggests that and individual’s sense of their objective memory is pertinent to episodic memory in general, and not selectively to associative memory.
The present results should be interpreted in the context of its limitations and strengths. First, although our study hypotheses included risk factors for AD, specifically APOE and amyloid, we did not prospectively recruit participants into the study based on their genetic or amyloid status. This led to uneven APOE groups, potentially decreasing power to detect subtle differences between those groups. APOE ε4 homozygotes are very rare, and heterozygotes relatively rare in the population, making these subgroups smaller, and potentially biased to be cognitively healthy enough for inclusion in a healthy aging study, as APOE ε4 is associated with an earlier onset of AD (Corder, 1994; Farrer et al., 1997). Similarly, the amyloid subsample consisted of individuals recruited from the whole sample that were 50 and older. Only 20% of adults over 60 show elevated levels of amyloid (Rodrigue et al., 2012) resulting in a smaller group of potential amyloid positive individuals to study. Larger subsamples are desirable to determine if these results generalize to the larger population, particularly our amyloid x sex interaction. Caution is warranted when interpreting small subgroup findings. Second, the current research utilized a cross-sectional approach and the tradeoffs of cross-sectional vs. longitudinal designs apply. Although we cannot directly measure individual change in episodic memory, our cross-sectional design allows for investigation of a wide age range of the adult lifespan. It will be important to follow these individuals over time to investigate whether, for example, increased amyloid accumulation is associated with greater memory complaints, and decline in associative memory performance. This might be especially important for the construct of subjective memory assessment, which is designed to detect perceived change within an individual.
Overall, the current study indicates that even within a cognitively healthy cross-section of the adult age-span, individual assessment of perceived decline in memory function is indicative of performance on laboratory based, objective measures of associative memory abilities. Subjective self-report of memory decline predicted associative memory recall after accounting for aging effects, sex differences, differences in depressive mood, and a major genetic risk factor for AD, APOE status. Our results indicate that difference in affect is not a primary explanatory factor of subjective memory assessment or its link to actual memory performance. We further illustrate that the link between subjective cognitive assessment and memory performance may be driven, in part, or enhanced by, the presence of AD risk factors such as APOE genotype or beta-amyloid deposition. Although further research is needed to determine how to successfully utilize SM complaints to identify at-risk populations, the present research suggests that perceived failures of memory may be early indicators of future problems.
Acknowledgments
This study was supported in part by National Institutes of Health grants R00-AG-36848 to Karen M. Rodrigue and R00-AG-36818 to Kristen M. Kennedy, and by an Investigator Initiated Trial grant from Lilly Pharmaceuticals/Avid Radiopharmaceuticals for the Amyvid ligand. We thank David Hoagey for assistance in MRI and PET image processing.
Footnotes
Portions of the current findings were presented at the Cognitive Aging Conference in Atlanta, GA, April 2016 and the Dallas Aging and Cognition conference in Dallas, TX, January 2017.
References
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67(9):1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla-Wilson K, Bleecker ML. Influence of verbal intelligence, sex, age, and education on the Rey Auditory Verbal Learning Test. Developmental Neuropsychology. 1986;2(3):203–211. [Google Scholar]
- Clarnette RM, Almeida OP, Forstl H, Paton A, Martins RN. Clinical characteristics of individuals with subjective memory loss in Western Australia: results from a cross-sectional survey. International Journal of Geriatric Psychiatry. 2001;16(2):168–174. doi: 10.1002/1099-1166(200102)16:2<168::aid-gps291>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small G, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NI, Strittmatterl WI, Schmechel DE. Protective effect of apolipoprotein E type 2 allele for late onset. Nature Genetics. 1994:7. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 51–110. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Derouesné C, Lacomblez L, Thibault S, Leponcin M. Memory complaints in young and elderly subjects. International Journal of Geriatric Psychiatry. 1999;14(4):291–301. [PubMed] [Google Scholar]
- Dik MG, Jonker C, Comijs HC, Bouter LM, Twisk JWR, Van Kamp GJ, Deeg DJH. Memory complaints and APOE-ε4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57(12):2217–2222. doi: 10.1212/wnl.57.12.2217. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, Van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. Journal of the International Neuropsychological Society. 2002;8(01):58–71. [PubMed] [Google Scholar]
- Geerlings MI, Jonker C, Bouter LM, Adèr HJ, Schmand B. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. American Journal of Psychiatry. 1999;156(4):531– 537. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- Geffen G, Moar KJ, O’hanlon AP, Clark CR, Geffen LB. Performance measures of 16–to 86-year-old males and females on the auditory verbal learning test. The Clinical Neuropsychologist. 1990;4(1):45–63. doi: 10.1080/13854049008401496. [DOI] [PubMed] [Google Scholar]
- Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for Assessment of Memory Complaints in Adulthood and Old Age. Psychology and Aging. 1990;5(4):482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Reisberg B, De Santi S, Babb JS, Pirraglia E, Rich KE, Brys M, de Leon MJ. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dementia and geriatric cognitive disorders. 2007;24(3):177–184. doi: 10.1159/000105604. [DOI] [PubMed] [Google Scholar]
- Golinkoff M, Sweeney JA. Cognitive impairments in depression. Journal of Affective Disorders. 1989;17(2):105–112. doi: 10.1016/0165-0327(89)90032-3. [DOI] [PubMed] [Google Scholar]
- Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54(4):827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- Hänninen T, Reinikainen KJ, Helkala EL, Koivisto K, Mykkänen L, Laakso M, Pyörälä K, Riekkinen PJ. Subjective Memory Complaints and Personality Traits in Normal Elderly Subjects. Journal of the American Geriatrics Society. 1994;4(42):1–4. doi: 10.1111/j.1532-5415.1994.tb06064.x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Nilsson LG, Bäckman L. Gender differences in episodic memory. Memory and Cognition. 1997;25(6):801–811. doi: 10.3758/bf03211324. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Airaksinen E, Nordström E. Sex differences in episodic memory: the impact of verbal and visuospatial ability. Neuropsychology. 1999;13(4):590. doi: 10.1037//0894-4105.13.4.590. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Park DC, Morrell RW, Martin M. Ask and ye shall receive: Behavioural specificity in the accuracy of subjective memory complaints. Applied Cognitive Psychology. 2000;14(3):257–275. [Google Scholar]
- Hertzog C, Pearman A. Memory complaints in adulthood and old age. In: Perfect TJ, Lindsay D, editors. The SAGE Handbook of Applied Memory. 1. Sage Publications; London: 2014. [Google Scholar]
- Hodges J. The amnestic prodrome of Alzheimer’s disease. A Journal of Neurology. 1998;121(9):1601–1602. doi: 10.1093/brain/121.9.1601. [DOI] [PubMed] [Google Scholar]
- Jacinto AF, Brucki SMD, Porto CS, de Arruda Martins M, Nitrini R. Subjective memory complaints in the elderly: a sign of cognitive impairment? Clinics. 2014;69(3):194–197. doi: 10.6061/clinics/2014(03)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. The Lancet Neurology. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker C, Launer LJ, Hooijer C, Lindeboom J. Memory complaints and memory impairment in older individuals. Journal of the American Geriatrics Society. 1996;44(1):44–49. doi: 10.1111/j.1532-5415.1996.tb05636.x. [DOI] [PubMed] [Google Scholar]
- Kahn RL, Zarit SH, Hilbert NM, Niederehe G. Memory Complaint and Impairment in the Aged: The Effect of Depression and Altered Brain Function. Archives of General Psychiatry. 1975 Dec;32(12):1569–1573. doi: 10.1001/archpsyc.1975.01760300107009. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Reese E, Horn M, Sizemore A, Unni A, Meerbrey M, Kalich A, Rodrigue KM. Polymorphism Affects Aging of Multiple Types of Memory. Brain Research, Special Issue on Memory and Aging. 2015;1612:104–117. doi: 10.1016/j.brainres.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws SM, Hone E, Taddei K, Harper C, Dean B, McClean C, Masters C, Lautenschlager N, Gandy SE, Martins RN. Variation at the APOE-491 promoter locus is associated with altered brain levels of apolipoprotein E. Molecular psychiatry. 2002;7(8):886. doi: 10.1038/sj.mp.4001097. [DOI] [PubMed] [Google Scholar]
- Light LL. The organization of memory in old age. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 111–165. [Google Scholar]
- Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, Kaplan EF, D’agostino RB. The ‘preclinical phase’ of probable Alzheimer’s disease: a 13-year prospective study of the Framingham cohort. Archives of Neurology. 1995;52(5):485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Roberts RO, Geda YE, Swenson-Dravis DM, Boeve BF, Senjem ML, Vemuri P, Petersen RC, Jack CR. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012;79(15):1570–1577. doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minett TS, Da Silva RV, Ortiz KZ, Bertolucci PH. Subjective Memory Complaints in an Elderly Sample: A Cross-sectional Study. International Journal of Geriatric Psychiatry. 2008;23(1):49–54. doi: 10.1002/gps.1836. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C] PIB in a nondemented population Potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, Rich KE, Switalski R, Mehta PD, Pratico D, Zinkowski R, Blennow K, De Leon MJ. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biological Psychiatry. 2008;63(6):609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(5):1170. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Hussain Z, Guez J, Bar-On M. Adult age differences in episodic memory: further support for an associative-deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29(5):826. doi: 10.1037/0278-7393.29.5.826. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative memory deficit of older adults: further support using face-name associations. Psychol Aging. 2004;19:541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Maddox GB, Jones P, Old S, Kilb A. The effects of emotional arousal and gender on the associative memory deficit of older adults. Memory & cognition. 2012;40(4):551–566. doi: 10.3758/s13421-011-0169-x. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Bäckman L, Erngrund K, Olofsson U, Nilsson LG. Age differences in episodic memory, semantic memory, and priming: Relationships to demographic, intellectual, and biological factors. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1996;51(4):P234–P240. doi: 10.1093/geronb/51b.4.p234. [DOI] [PubMed] [Google Scholar]
- O’Hara R, Yesavage JA, Kraemer HC, Mauricio M, Friedman LF, Murphy GM. The APOEε 4 allele Is Associated with Decline on Delayed Recall Performance in Community-Dwelling Older Adults. Journal of the American Geriatrics Society. 1998;46(12):1493–1498. doi: 10.1111/j.1532-5415.1998.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17(2):299. [PubMed] [Google Scholar]
- Parisi JM, Gross AL, Rebok GW, Saczynski JS, Crowe M, Cook SE, Langbaum J, Sartori A, Unverzagt FW. Modeling change in memory performance and memory perceptions: Findings from the ACTIVE study. Psychology and aging. 2011;26(3):518. doi: 10.1037/a0022458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearman A, Hertzog C, Gerstorf D. Little evidence for links between memory complaints and memory performance in very old age: Longitudinal analyses from the Berlin Aging Study. Psychology and Aging. 2014;29(4):828. doi: 10.1037/a0037141. [DOI] [PubMed] [Google Scholar]
- Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Archives of Neurology. 2012;69(2):223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Polcher A, Frommann I, Koppara A, Wolfsgruber S, Jessen F, Wagner M. Face-name associative recognition deficits in subjective cognitive decline and mild cognitive impairment. Journal of Alzheimer’s Disease. 2017;56(3):1185–1196. doi: 10.3233/JAD-160637. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reifler BV, Larson E, Hanley R. Coexistence of cognitive impairment and depression in geriatric outpatients. The American Journal of Psychiatry. 1982;139(5):623–626. doi: 10.1176/ajp.139.5.623. [DOI] [PubMed] [Google Scholar]
- Revell AJ, Caskie GIL, Willis SL, Schaie KW. Replication and exploration of factor solutions for the Memory Functioning Questionnaire (MFQ) in older adults. Gerontologist. 2001 Oct;41:133–133. [Google Scholar]
- Riedel-Heller SG, Matschinger H, Schork A, Angermeyer MC. Do memory complaints indicate the presence of cognitive impairment? –Results of a field study. European Archives of Psychiatry and Clinical Neuroscience. 1999;249(4):197–204. doi: 10.1007/s004060050087. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Rieck JR, Hebrank AC, Diaz-Arrastia R, Matthews D, Park DC. β-Amyloid burden in healthy aging Regional distribution and cognitive consequences. Neurology. 2012;78(6):387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46(1):121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- Schmand B, Jonker C, Geerlings MI, Lindeboom J. Subjective memory complaints in the elderly: depressive symptoms and future dementia. The British Journal of Psychiatry. 1997;171(4):373–376. doi: 10.1192/bjp.171.4.373. [DOI] [PubMed] [Google Scholar]
- Small BJ, Herlitz A, Fratiglioni L, Almkvist O, Bäckman L. Cognitive predictors of incident Alzheimer’s disease: a prospective longitudinal study. Neuropsychology. 1997;11(3):413. doi: 10.1037//0894-4105.11.3.413. [DOI] [PubMed] [Google Scholar]
- Small GW, Chen ST, Komo S, Ercoli L, Miller K, Siddarth P, Kaplan A, Dorsey D, Lavretsky, Saxena S, Bookheimer SY. Memory self-appraisal and depressive symptoms in people at genetic risk for Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2001;16(11):1071–1077. doi: 10.1002/gps.481. [DOI] [PubMed] [Google Scholar]
- Smith GE, Petersen RC, Ivnik RJ, Malec JF, Tangalos EG. Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychology and Aging. 1996;11(2):272. doi: 10.1037//0882-7974.11.2.272. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron. 2014:608–622. doi: 10.1016/j.neuron.2014.10.038. z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Russ C, Richards M, Brayne C, Lovestone S, Mann A. Depression, APOE Genotype and Subjective Memory Impairment: A Cross- sectional Study in an African-Caribbean Population. Psychological Medicine. 2001;31(3):431–440. [PubMed] [Google Scholar]
- Vannini P, Amariglio R, Hanseeuw B, Johnson KA, McLaren DG, Chhatwal J, Pascual-Leone A, Rentz D, Sperling RA. Memory self-awareness in the preclinical and prodromal stages of Alzheimer’s disease. Neuropsychologia. 2017;99:343–349. doi: 10.1016/j.neuropsychologia.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: A quantitative integration of research findings. Journal of gerontology. 1993;48(4):P157–P171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Wang L, Van Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, Larson EB. Subjective memory deterioration and future dementia in people aged 65 and older. Journal of the American Geriatrics Society. 2004;52(12):2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- Weber M, Mapstone M, Staskiewicz J, Maki PM. Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause (New York, NY) 2012;19(7):735. doi: 10.1097/gme.0b013e318241fd22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Administration and Scoring Manual for the Wechsler Memory Scale. Pearson; San Antonio, TX: 2009. [Google Scholar]
- West RL, Crook TH, Barron KL. Everyday memory performance across the life span: effects of age and noncognitive individual differences. Psychology and Aging. 1992;7(1):72. doi: 10.1037//0882-7974.7.1.72. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N, Schrank FA. Woodcock– Johnson III Diagnostic Supplement to the Tests of Cognitive Abilities. Riverside Publishing; Itasca, IL: 2003. [Google Scholar]
- Zelinski EM, Gilewski MJ, Schaie KW. Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychology and Aging. 1993;8(2):176. doi: 10.1037//0882-7974.8.2.176. [DOI] [PubMed] [Google Scholar]