Figure 1.
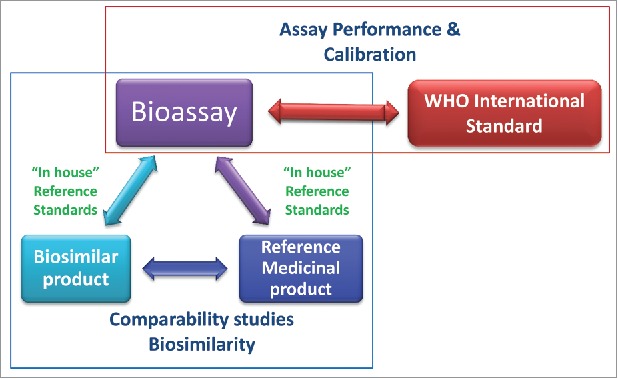
The roles of the WHO international standards (IS) and the reference medicinal product (RMP) in relation to the bioassay. The WHO IS supports bioassay performance and calibration. Bioassays are used as part of the comparability studies to demonstrate biosimilarity between the biosimilar product and the RMP.
