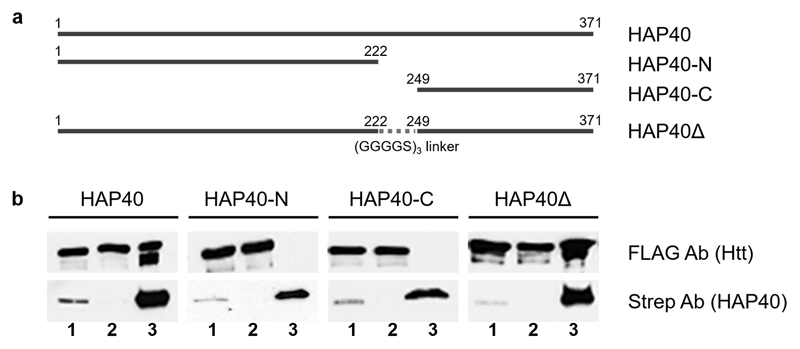
Extended Data Fig. 6. Truncation analysis of HAP40 binding to Htt.
a, Schematic representation of the HAP40 constructs studied (all C-terminally Strep-tagged). b, HAP40 constructs were co-expressed with C-terminally FLAG-tagged 17QHtt, immunoprecipitated using Strep-Tactin beads and analyzed by Western blot. Lanes are indicated as follows: 1, cell lysates; 2, cell lysates upon incubation with Strep beads; 3, Strep beads eluates. Note that full-length HAP40 and a construct lacking the central domain immunoprecipitate Htt, but not deletions of the N- and C-terminal regions of HAP40. Independent experiments with similar results (n): n=2. For gel source data, see Supplementary Figure 1.