Table 1.
Evaluation of Chiral Copper Complexesa
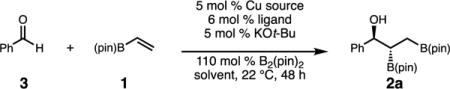
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| entry | ligand | Cu source | solvent | NMR yield (%)b | drb | erc,d |
| 1 | L1 | Cu(MeCN)4PF6 | thf | 87 | 6.7:1 | 77:23 |
| 2 | L1 | Cu(MeCN)4PF6 | toluene | >98 | 4.6:1 | 82:18 |
| 3 | L2 | Cu(MeCN)4PF6 | toluene | 98 | 1.7:1 | 92:8 |
| 4 | L3 | Cu(MeCN)4PF6 | toluene | 88 | 1:1 | 95:5 |
| 5 | L4 | Cu(MeCN)4PF6 | toluene | <2 | – | – |
| 6 | L5 | Cu(MeCN)4PF6 | toluene | <2 | – | – |
| 7 | L6 | Cu(MeCN)4PF6 | toluene | 12 | 11.5:1 | – |
| 8 | L7 | Cu(MeCN)4PF6 | toluene | <2 | – | – |
| 9 | L8 | Cu(MeCN)4PF6 | toluene | >98 | 3.2:1 | 95:5 |
| 10 | L8 | CuOt-Bu | toluene | 87 | 3:1 | 95:5 |
| 11e | L8 | CuOt-Bu | C6H6 | >98 | 3.5:1 | 95.5:4.5 |
|
| ||||||
| Ligands: | ||||||
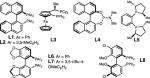
| ||||||
Reactions performed under N2 atmosphere.
Determined by analysis of 400 or 600 MHz 1H NMR spectra of crude reactions with hexamethyldisiloxane as internal standard.
Determined by HPLC analysis; see the Supporting Information for details.
The er of the syn diastereoisomer is not reported due to its propensity to undergo elimination.
[0.167 M] in benzene with 2 equivalents of 1.
