Table 2.
Enantioselective Cu-Catalyzed Multicomponent Borylation/1,2-Addition of Vinyl-B(pin) to Aryl, Alkenyl and Alkyl Aldehydesa
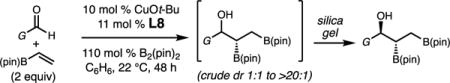
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | product | isolated yield (%)d | isolated dr (anti syn)b | erc | |
| 1 | 2a | G = Ph | 74 | >20:1 | 96:4 |
| 2 | 2b | G=p-FC6H4 | 70 | >20:1 | 96:4 |
| 3 | 2c | G = p-BrC6H4 | 68 | 5.7:1 | 95:5 |
| 4 | 2d | G = p-OMeC6H4 | 70 | >20:1 | 94:6 |
| 5e | 2e | G =m-MeC6H4 | 75(77) | >20:1 (4:1) | 95:5 |
| 6 | 2f | G = o-MeC6H4 | 81 | >20:1 | 96:4 |
| 7 | 2g | G = o-BrC6H5 | 83 | >20:1 | 96:4 |
| 8 | 2h |
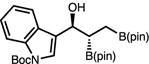
|
63 | 3:1 | 94:6 |
| 9e | 2i |
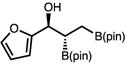
|
52 (57) | >20:1 (3.5:1) | 97:3 |
| 10 | 2j |
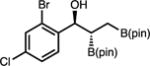
|
71 | >20:1 | 95:5 |
| 11 | 2k |
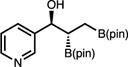
|
18t | – | – |
| 12 | 2I |
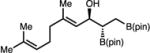
|
78 (36) | >20:1 (>20:1 dr) | 93:7 |
| 13 | 2m |
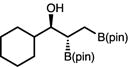
|
63 | >20:1 | 92:8 |
| 14 | 2n |
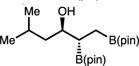
|
48 | >20:1 | 75:25 |
| 15e | 2o |
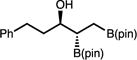
|
70t (52) | 3:1 (3:1) | 90:10 |
See Table 1.
Isolated yield of the anti diastereoisomer unless otherwise stated.
Values in parentheses correspond to the TBS protected hydroxydiboronate, resulting from protection of the crude reaction mixture (see Supporting Information for details).
NMR yield.
