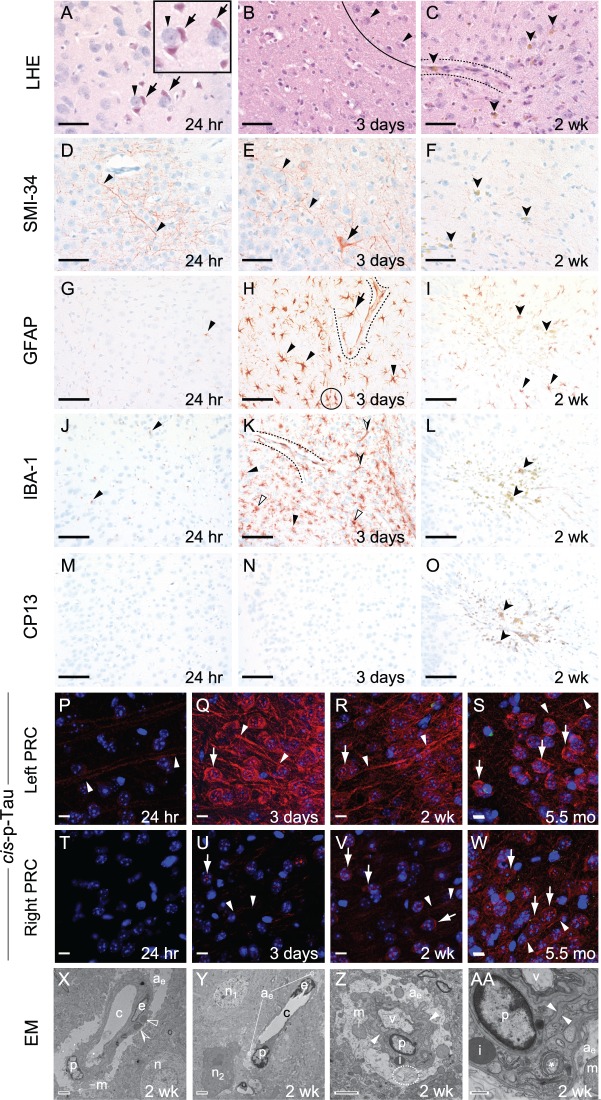
Figure 3.
Experimental closed-head injury induces early and progressive brain pathologies associated with CTE in cerebral cortex ipsilateral and subjacent to impact. (A–C) Luxol fast blue haematoxylin and eosin (LHE) staining in ipsilateral (left) perirhinal cortex 24 h (A), 3 days (B), 2 weeks (C) post-injury. (A) LHE staining at 24 h post-injury revealed dying neurons (arrows) with pyknotic basophilic nuclei and intensely eosinophilic cytoplasm interspersed with normal-appearing neurons (arrowheads). Black box, magnified view showing neuronal necrosis. Scale bar = 100 μm. (B) Decreased neuronal density (below line) indicative of neuronal demise, ipsilateral (left) perirhinal cortex 3 days post-injury. Normal-appearing neurons (arrowheads, above line). Scale bar = 100 μm. (C) Decreased neuronal density (below line) indicative of neuronal demise and gliosis near a small blood vessel (between dashed lines). Clusters of haemosiderin-laden macrophage (darts) represent microhaemorrhage residua. Scale bar = 100 μm. Contralateral (right) perirhinal cortex was histopathologically normal by LHE staining (Supplementary Fig. 2A–C). (D–F) Immunostaining with monoclonal antibody SMI-34 (phosphorylated neurofilament) in ipsilateral (left) perirhinal cortex 24 h (D), 3 days (E), 2 weeks (F) post-injury. (D and E) SMI-34 immunostaining revealed neurons with swollen, beaded neuronal processes (arrowheads) and cytoplasmic immunoreactivity (arrow, E). Scale bars = 100 μm. (F) Haemosiderin-laden macrophages (darts), but not SMI-34 immunoreactivity, were observed in the ipsilateral (left) perirhinal cortex 2 weeks post-injury. Scale bar = 100 μm. Contralateral (right) perirhinal cortex was histopathologically normal by SMI-34 immunostaining (Supplementary Fig. 2D–F). (G–I) Immunostaining for astrocytic glial fibrillary acidic protein (GFAP) in ipsilateral (left) perirhinal cortex 24 h (D), 3 days (E), 2 weeks (F) post-injury. (G) Sparse GFAP-immunoreactivity (arrowhead) was present 24 h post-injury. Scale bar = 50 μm. (H) Brisk reactive astrocytosis at 3 days post-injury. Clusters of hypertrophied GFAP-immunopositive reactive astrocytes (arrowheads) with ramified processes and perivascular astrocytes (arrow) with hydropic end-feet terminating on small blood vessels (dashed lines) were present 3 days post-injury. Overlapping astrocytic processes (black circle) indicate disruption of domain restriction. Scale bars = 50 μm. (I) Reactive astrocytes (arrowheads) were present 2 weeks post-injury. Haemosiderin-laden macrophages (darts), representing microhaemorrhage residua, were scattered throughout the affected region. Scale bar = 50 μm. Contralateral (right) perirhinal cortex was histopathologically normal by GFAP immunostaining (Supplementary Fig. 2G–I). (J–L) Immunostaining for the myeloid cell marker Iba1 (arrowheads) in ipsilateral (left) perirhinal cortex revealed minimal microgliosis at 24 h (J), brisk microgliosis at 3 days (K), resolved microgliosis at 2 weeks (L) post-injury. Scale bars = 50 μm. (K) Peak microgliosis at 3 days post-injury revealed clusters of intensely Iba1-immunoreactive, ramified myeloid cells (arrowheads) and less abundant amoeboid and rodlike Iba1-immunoreactive microglia (open and half-filled arrowheads, respectively). Iba1-immunoreactive perivascular myeloid cells were associated with the parenchymal (abluminal) surface of a small blood vessel (between dashed lines). Scale bar = 50 μm. (L) Microgliosis was largely resolved by 2 weeks post-injury. Haemosiderin-laden macrophage (darts) were observed throughout the affected region. Scale bar = 50 μm. Contralateral (right) perirhinal cortex was histopathologically normal by Iba1 immunostaining (Supplementary Fig. 2J–L). (M–O) Phosphorylated tau protein immunostaining with monoclonal antibody CP13 (pS202) was negative throughout the brain at 24 h (M), 3 days (N), and 2 weeks (O) post-injury. Haemosiderin-laden macrophage (darts) were observed throughout the affected region by 2 weeks post-injury (O). Scale bars = 50 μm. The contralateral (right) perirhinal cortex did not demonstrate CP13 immunostaining (Supplementary Fig. 2M–O). (P–W) Immunohistofluorescence staining for cis-p-tau (cis-motif, pThr231-Pro), a highly pathogenic early phosphorylated tau proteoform, was present at 24 h (P), 3 days (Q), 2 weeks (R), and 5.5 months (S) post-injury in ipsilateral (left) perirhinal cortex. Faint cis-p-tau immunoreactivity was observed in axons (arrowheads) in the ipsilateral (left, P) but not contralateral (right, T) perirhinal cortex 24 h post-injury. By 3 days post-injury, cis-p-tau immunoreactivity in the ipsilateral perirhinal cortex (Q) was intense, not only in axons (arrowheads), but also as dot-like inclusions in neuronal soma and dendrites (arrow). Cis-p-tau immunoreactivity in the contralateral perirhinal cortex (U) was present but faint at this time point. By 2 weeks post-injury, cis-p-tau immunoreactivity was observed in axons (arrowheads) and as dot-like inclusions in neuronal soma and dendrites (arrow) in both hemispheres. Cis-p-tau immunoreactivity was more pronounced in the ipsilateral (left, R) than contralateral (right, V) perirhinal cortex. Surprisingly, we detected cis-p-tau immunoreactivity 5.5 months post-injury (the longest time point measured) in axons (arrowheads) and as dot-like inclusions in neuronal soma and dendrites (arrow) in perirhinal cortex of both hemispheres (left, S; right, W). Scale bars = 20 µm. Sham (no-injury) control mice did not show evidence of cis-p-tau immunoreactivity in either hemisphere at any of the analysed time points (Supplementary Fig. 2P–W). (X–AA) Ultrastructural evidence of persistent traumatic microvascular injury revealed by electron microscopy 2 weeks post-injury. (X) Low-power electron micrograph of CA1 region of the left hippocampus shows abnormal capillary (c) and nearby neuron (n). A capillary endothelial cell (e) and adjacent pericyte (p) are encircled by hydropic astrocytic end-feet (ae). Perivascular astrocyte processes exhibit pale oedematous cytoplasm with few mitochondria (m), subcellular organelles, or cytoskeletal elements. The capillary basal lamina is thickened, highly branched, and tortuous. Electron-dense inclusion bodies (open arrowheads) and lipofuscin granules with lipid droplets (partially-filled arrowhead) are evident. These ultrastructural pathologies are inconsistent with processing artefact. Magnification ×1500. Scale bar = 2 µm. (Y) Low-power electron micrograph of left medial prefrontal cortex 2 weeks after injury shows an abnormal capillary (c) and two nearby neurons, one with normal ultrastructure (n1) and the other undergoing cellular involution (n2). Endothelial cell (e), perivascular pericyte (p), and hydropic astrocytic end-feet (ae) are present. Magnification ×1200. Scale bar = 2 µm. (Z) Electron micrograph of the CA1 region of the left hippocampus shows a hydropic astrocytic end-foot (ae) and pericyte (p) of an involuting capillary. The basal laminae are thickened and tortuous (arrowheads). Electron-dense inclusion body (i), swollen mitochondria (m), and autophagosomic vacuoles (v, dashed ellipse) are present. Magnification ×3000. Scale bar = 2 µm. (AA) High magnification electron micrograph of the same CA1 region of the left hippocampus showing ultrastructural details of the hydropic astrocytic end-foot (ae) and pericyte (p). Thickened, tortuous basal lamina (arrowheads), inclusion body (i), swollen mitochondrion (m), and degenerating mitochondrion (asterisk) are present. Magnification ×10 000. Scale bar = 500 nm. n = 4 mice per group.