Abstract
Exposure to fine particulate matter (PM) air pollution causes adverse cardiopulmonary outcomes. Yet, the limited capacity to readily identify contributing PM sources and associated PM constituents in any given ambient air shed impedes risk assessment efforts. The health effects of PM have been attributed in part to its capacity to elicit irritant responses. A variety of chemicals trigger irritant behavior responses in zebrafish that can be easily measured. The purposes of this study were to examine the utility of zebrafish locomotor responses in the toxicity assessment of fine PM and its chemical fractions and uncover mechanisms of action. Locomotor responses were recorded in 6-day-old zebrafish exposed for 60 min in the dark at 26 °C to the extractable organic matter of a compressor-generated diesel exhaust PM (C-DEP) and 4 of its fractions (F1–F4) containing varying chemical classes of increasing polarity. The role of the transient receptor potential (TRP) cation channel TRPA1, a chemical sensor in mammals and zebrafish, in locomotor responses to C-DEP, was also examined. Acrolein, an environmental irritant and known activator of TRPA1, and all extracts induced concentration-dependent locomotor responses whose potencies ranked as follows: polar F3 > weakly polar F2 > C-DEP > highly polar F4 > nonpolar F1, indicating that polar and weakly polar fractions that included nitro- and oxy-polyaromatic hydrocarbons (PAHs), drove C-DEP responses. Irritant potencies in fish positively correlated with mutagenic potencies of the same extracts in strains of Salmonella sensitive to nitro- and oxy-PAHs, further implicating these chemical classes in the zebrafish responses to C-DEP. Pharmacologic inhibition of TRPA1 blocked locomotor responses to acrolein and the extracts. Taken together, these data indicate that the zebrafish locomotor assay may help expedite toxicity screening of fine PM sources, identify causal chemical classes, and uncover plausible biological mechanisms.
Keywords: air pollution, particulate matter, zebrafish, locomotor, irritant responses, TRPA1
In a recent global survey of chronic disease, exposure to particulate matter (PM) air pollution was the ninth leading risk factor, driven largely by cardiovascular and respiratory disease outcomes (Lim et al., 2012). Although ambient PM mass and size are major determinants of PM-related adverse health effects, leachable components of PM, including metals, and polyaromatic hydrocarbons (PAHs), are the key initiators of PM-induced biological responses (Brook et al., 2010; Thomson et al., 2016; Xia et al., 2004), and are associated with measurable clinical effects apart from those attributable to PM mass (Delfino et al., 2010; Kraus et al., 2011; Ostro et al., 2007; Ruiz-Vera et al., 2015; Wittkopp et al., 2016; Wu et al., 2016; Zhang et al., 2016). PM composition, however, is exceedingly complex, varying considerably across air sheds, and heavily influenced by weather, time of day, and proximity to emission sources (Brook et al., 2010). Thus, information on the relative contribution of various PM sources and associated PM constituents in the toxicity of ambient PM is limited or unavailable, impeding risk assessment.
The most common experimental approaches used to assess the in vivo toxicity of PM sources are rodent inhalation and instillation studies, which are constrained by requirements for large amounts of PM, often in limited supply, and are low throughput. Higher throughput in vitro methods using mammalian cells fail to recapitulate the complexity of an intact organism, including the crosstalk of organ systems and the resulting multisystem physiological responses elicited by PM exposure. The zebrafish (Danio rerio), a fully intact vertebrate in vivo model, is a higher throughput alternative to mammalian models, with a high degree of functional conservation with mammals (Peterson and MacRae, 2012), and offers technical simplicity on par with in vitro cell assays. Moreover, zebrafish skin is sensitive to many chemicals known to irritate mammalian lung epithelium, including the aldehyde acrolein (Prober et al., 2008), and expresses the transient receptor potential (TRP) cation channel TRPA1, which like its mammalian ortholog, mediates chemosensation to exogenous and endogenous agents (Prober et al., 2008), as well as chemicals that cause oxidative stress (Takahashi and Mori, 2011). Importantly, PM is rich in chemical triggers of oxidative stress including PAHs (Baulig et al., 2003), and TRPA1 has been described as a major sensor of oxidative stress in rodent pulmonary airways (Bessac et al., 2008).
The purpose of this study was to examine the utility of zebrafish locomotor behavior responses in the toxicity assessment of fine PM. Locomotor responses in zebrafish, wherein the distance moved reflects the potency of the irritant (Padilla et al., 2011; Prober et al., 2008), have been previously measured in response to a variety of chemicals, including bisphenol A (Wang et al., 2015), organophosphate flame retardants (Jarema et al., 2015; Oliveri et al., 2015; Sun et al., 2016), benzo[a]pyrene (Gao et al., 2015), atrazine (Liu et al., 2016), and crude oil (Perrichon et al., 2016). In this study, zebrafish larvae were exposed to a whole organic extract or 1 of 4 chemical fractions of 1 PM sample, ie, compressor-generated diesel exhaust PM (C-DEP), all of which were previously analyzed for organic chemical content (Mutlu et al., 2013). Diesel exhaust (DE) PM is a major contributor to traffic-derived ambient PM and is mostly composed of inorganic carbon and a variety of organic compounds such as PAHs and nitroarenes (nitro-PAHs) (IARC, 2014). C-DEP has been previously analyzed for mutagenicity (Mutlu et al., 2013), electrophilic and redox properties (Shinyashiki et al., 2009), and induction of matrix metalloproteinases in human bronchial epithelial cells (Li et al., 2009). By evaluating chemical fractions of C-DEP, we inferred which chemical classes were most responsible for the irritant responses. We also examined the role of TRPA1 in irritant responses to C-DEP in zebrafish, given that TRPA1-mediated adverse cardiac responses to DE in rodents (Hazari et al., 2011) and TRPA1 in mammalian cells in vitro is activated by various DE PM chemicals (Deering-Rice et al., 2011).
MATERIALS AND METHODS
Chemicals and particulate extracts
Dimethyl sulfoxide (DMSO) was obtained from Sigma Aldrich (St Louis, Missouri). Acrolein was obtained from O2SI Smart Solutions (Charleston, South Carolina). The TRPA1 antagonist HC030031 was obtained from Tocris Bioscience (Ellisville, Missouri). C-DEP was generated, extracted, and fractionated as previously described (Mutlu et al., 2013); the same extracts prepared by Mutlu et al. (2013), stored frozen in DMSO at 4°C, were used for this study. Previous studies have shown that diesel exhaust extracts are highly stable after many years of storage, eg, National Institute of Standards and Technology standard SRM1650 had almost identical mutagenic potencies in strains TA98 and TA100 of Salmonella after 5 years of storage (Claxton et al., 1992; Hughes et al., 1997). Briefly, C-DEP was produced by combusting low-sulfur petroleum diesel in a 30-kW (40 hp) 4-cylinder Deutz BF4M1008 diesel engine; particles were collected in a bag house and stored in sealed glass containers under nitrogen at 4°C until extracted by Mutlu et al. (2013). The particles were extracted by sonication with dichloromethane (DCM), and the DCM whole extract was fractionated sequentially on a silica-gel column with hexane (nonpolar fraction 1 [F1]), 75% hexane: 25% DCM (weakly polar fraction 2 [F2]), DCM (polar fraction 3 [F3]), and methanol (highly polar fraction 4 [F4]; Table 1). The percent extractable organic matter (EOM) was determined gravimetrically, and the extracts were solvent-exchanged into DMSO at 10 mg EOM/ml for the whole extract and 250 µg EOM/ml for the 4 fractions. Note: gravimetric analysis showed that the % EOM of the organic (DCM) extract of the C-DEP particle was 23%, which means that 23% of the weight of the particles was extractable by DCM, and it was this organic material (ie, whole C-DEP extract) that was fractionated.
Table 1.
Chemical Characteristics of C-DEP Fractionsa
| Fraction 1 | Fraction 2 | Fraction 3 | Fraction 4 | |
|---|---|---|---|---|
| Polarity | Nonpolar | Weakly polar | Moderately polar | Highly polar |
| Example compounds | Alkanes/alkenes | PAHs and nitro-PAHs | Nitro- and oxy-PAHs | Oxy-PAHs |
| % EOM of C-DEP | Approximately 52 | Approximately 5 | Approximately 15 | Approximately 28 |
Adapted from Mutlu et al. (2013).
Experimental animals
Wild type adult zebrafish (D. rerio; undefined, outbred stock originally obtained from Aquatic Research Organisms, Hampton, New Hampshire 03842 and EkkWill Waterlife Resources Ruskin, Florida 33575) were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facility with a 14:10 h light:dark cycle (lights on at 08:30 h). We have previously determined (unpublished data) that behavioral responses in our outbred line of fish are very similar to those in the AB line of fish. Adult fish (males and females housed together, approximately 8 per liter) were kept in 1 of several 9-liter (l) colony tanks (Aquaneering Inc, San Diego, California) with a water temperature of 28°C. For group spawning, all the adults from 2 different home tanks (approximately 80–100 total fish) were placed in 15 l static tanks set up approximately 3 pm, with embryos collected the next morning between 8:30 and 9 am. All embryos were gathered from the breeder tank and placed in a 26°C water bath and washed twice with 0.06% bleach (vol/vol) in 10% Hanks’ Balanced Salt Solution (HBSS; consisted of a mix of NaCl, KCl, Na2HPO4, CaCl2, MgSO4, and NaHCO3, all chemicals from Sigma Aldrich) for 5 min.
All studies were carried out in accordance with the guidelines of, and approved by, the Institutional Animal Care and Use Committee at the U.S. Environmental Protection Agency’s National Health and Environmental Effects Research Laboratory.
Group size determinations and controlling for plate variability
We performed sample size analysis using R Studio software (R Studio, Inc) with the “pwr” package (https://cran.r-project.org/web/packages/pwr/pwr.pdf) and “pwr.anova.test” command and group sizes were calculated based on effect size indexes obtained from pilot studies. We performed all experiments with duplicate plates, and we combined data from both plates from each experiment only after ensuring that there were no plate-to-plate differences. A series of control plates were also run to compare the impact of the various vehicles (1) HBSS versus 0.4% DMSO and (2) 0.4% DMSO versus 0.8% DMSO.
Concentration-response studies with acrolein, whole C-DEP, and C-DEP fractions
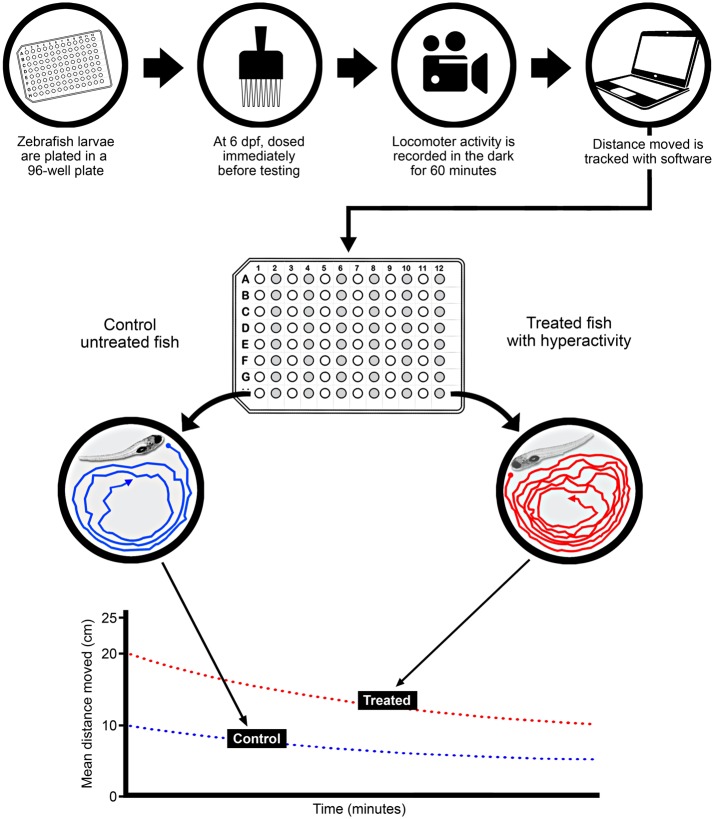
For the dose-response studies, embryos were plated into single wells of a 96-well plate (1 fish/well) on day 0 and then at 6-days postfertilization (dpf; n = 15–32/experimental condition) acutely exposed to vehicle (0.4% DMSO, final nominal concentration), a positive control acrolein (1–30 μM), whole C-DEP extract (0.125–40 µg/ml, final nominal concentrations in wells), or fractions 1 thru 4 (0.125–40 µg/ml, final nominal concentrations in wells) and activity was assessed in the dark at 26°C for 60 min using Noldus (Leesburg, Virginia) Ethovision video tracking software (Figure 1). Working stock solutions of C-DEP and F1 thru F4 in DMSO and of acrolein in high-performance liquid chromatography-grade water were prepared prior to dosing. Dosing was achieved by adding 1 μl of test solution to 250 μl of HBSS media in each well of a 96-well plate.
Figure 1.
Experimental protocol for treatment and behavior assessment of zebrafish larvae. Zebrafish were plated at 6–8 h postfertilization, and then dosed at 6-days postfertilization (dpf), immediately before assessment. Locomotor activity was recorded in the dark for 60 min, and analysis software was used to derive distance moved (cm)/unit time (min). Control (unshaded wells) and treated (shaded wells) fish were dosed in alternating columns of each 96-well plate.
For the whole extract and 4 fractions, we performed linear regressions over the linear portion of the dose-response curves to determine slopes, which were used to indicate the irritant potency of each extract and were expressed as mean distance moved (cm)/μg EOM/ml. This mirrors a similar approach used previously to quantify the mutagenic potencies of the same PM extracts (Mutlu et al., 2013). Slopes were selected based on the following criteria: (1) correlations that provided the highest goodness of fit (ie, r2 value) and (2) the highest concentration before the concentration curve plateaued.
TRPA1 antagonism studies
Locomotor activity was also measured in acrolein, C-DEP, F2, or F3-exposed zebrafish pretreated with a TRPA1 antagonist (HC030031). One microliter of HC030031 in 0.4% DMSO (final well-concentration of HC030031 = 50 µM) or 0.4% DMSO alone were added to each well 15 min before dosing with acrolein, C-DEP, F2, or F3. The TRPA1 antagonist pretreatment regimen and treatment concentration were adapted from Curtright et al. (2015). For the antagonism studies, final DMSO concentration was 0.8% for all wells because DMSO was used as the vehicle for both the TRPA1 antagonist and the PM extracts, which were administered separately.
Behavioral testing
All testing was performed on 6 dpf larvae in 96-well plates (Figure 1). Video recording of fish behavior was essentially as described previously (MacPhail et al., 2009).
On the morning of testing, after the rearing solution was changed, plates were moved to the dark in the behavioral testing room for 1 h. Temperature in the testing room was kept at 26°C. For testing, the plate was transferred to the light box for 25 min, with dosing taking place during the last 5 min of the light program. Because we were interested in irritant effects of exposure and because organisms often adapt to irritant responses over time, the dark program was started immediately after dosing using a behavior-recording system (Noldus Information Technology, Leesburg, Virginia) to record immediate irritant-induced movement of each fish for 60 min in response to treatment.
Analysis of fish movement
Fish movement (locomotion) was tracked from videos using Ethovision XT (Noldus Information Technology) software Version 8.5. Tracking rate was 5 samples/s (ie, an image was captured every 200 ms). A dynamic subtraction method was used to detect objects that were darker than background, with a minimum object size of 10 pixels. Tracks were analyzed for total distance moved (cm). The software provides a distance output per 2 min period. An input filter of 0.135 cm (minimum distance moved) was used to remove system noise. All locomotion data are expressed as distance moved (cm) per unit time (Figure 1). We calculated slopes from linear regressions of these data to determine the irritant potency (mean distance moved [cm]/µg EOM) of each extract.
Statistics
Data were analyzed as follows: (1) data (2 min locomotor activity over time) presented in Supplementary Figure 1 were assessed using a repeated ANOVA with time and dose as the independent variables and locomotor activity (distance moved/time) as the dependent variable using GraphPad Prizm Software Version 6.0 (La Jolla, California). Significance was set at P ≤ .05. (2) Data (10 min locomotor activity over time) presented in Figures 2–4 were first assessed using a repeated ANOVA with time and dose as the independent variables and locomotor activity (distance moved/time) as the dependent variable using Statview (SAS Institute, Inc, Cary, North Carolina; version 5.0.1). Significance was set at P ≤ .05. In the case of a significant time × dose interaction, step-down ANOVAs were performed to assess lower order effects. This involved first assessing if there was a significant effect of dose at each 10 min behavioral interval, and if so, Fisher’s Protected Least-Squares Difference comparisons were conducted to compare among dosage groups. (3) Linear regressions of dose-response data for whole C-DEP and F1–F4 (Table 2) and of plots of irritant potency in zebrafish versus mutagenic potency data (Table 3) were performed using GraphPad Prizm Software Version 6.0. For a comparison of the potency (ie, slope) data in Table 2, the slopes were compared via 1-way ANOVA followed by Tukey’s post hoc test (note: for this analysis, the slopes derived from the linear regression of the concentration-response curve for each extract were entered as the mean, the standard error for the slope also derived from the linear regression data was entered as the SEM, and the degree of freedom [df] value was entered as “n” or group size—the df for all groups in this analysis was 1). (4) Data (60-min locomotor activity) presented in Figure 5 was first assessed using an ANOVA with extract and antagonist treatment as the independent variables and locomotor activity (mean distance moved [cm] in 60 min) as the dependent variable using Statview (SAS Institute, Inc; version 5.0.1). If there was a significant interaction between extract and antagonist treatment, step-down ANOVAs were done to assess which treatment groups were different from one another. Significance was set at P ≤ .05. All data are presented as mean ± SEM. The number of independent observations are provided in the figure legends.
Figure 2.
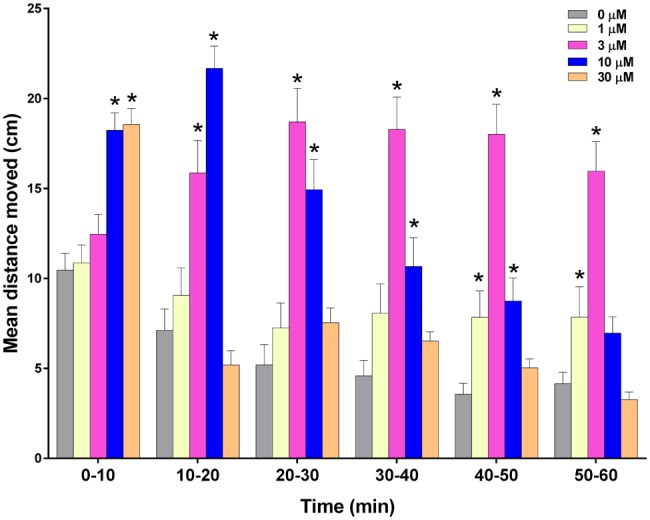
Effect of acrolein on zebrafish larval locomotor activity measured in the dark. Zebrafish were treated with 1, 3, 10, or 30 μM acrolein in 0.4% dimethyl sulfoxide (DMSO) or 0.4% DMSO alone approximately 5 min before monitoring locomotor behavior. Data are presented as mean ± SEM for the entire 60 min of recording. Data from 2 replicate plates were combined for a total of 31–32 zebrafish per treatment group. *Significantly greater than vehicle alone at corresponding time (P < .05).
Table 2.
Potency of C-DEP and Fractions in the Zebrafish Locomotor Assay
| Sample | Potency/Slopesa | r2 | P-Value* |
|---|---|---|---|
| C-DEP | 2.43 | 0.224 | < .0001 |
| F1 | 0.11 | 0.036 | .0024 |
| F2 | 4.96b | 0.175 | < .0001 |
| F3 | 11.35c | 0.169 | < .0001 |
| F4 | 1.79 | 0.237 | < .0001 |
Data are slopes of linear regressions of dose-response data.
The difference in potency between F2 and F1 approached statistical significance (P = .18).
Potency of F3 statistically greater than potency of C-DEP (P = .02), F1 (P = .01), and F4 (P = .02) and nearly F2 (P = .07).
Indicate P-values of linear regressions.
Table 3.
Correlations Between Irritant Potencies in Zebrafish and Mutagenic Potencies in Salmonellaa in Response to the Same Extracts
| C-DEPb | F1b | F2b | F3b | F4b | r2 for Fish Potency Versus Mutagenic Potency/Strain | P-Value | |
|---|---|---|---|---|---|---|---|
| Potency in zebrafish (cm/μg EOM/ml) | 2.43 | 0.11 | 4.96 | 11.35 | 1.79 | — | — |
| Mutagenic potency in TA100-S9c (Rev./μg EOM) | 13.10 | 0.00 | 41.30 | 60.40 | 8.00 | 0.93 | .0085 |
| Mutagenic potency in TA98-S9d (Rev./μg EOM) | 7.50 | 0.00 | 18.00 | 23.20 | 4.30 | 0.88 | .019 |
| Mutagenic potency in TA98NR-S9e (Rev./μg EOM) | 4.70 | 0.00 | 12.80 | 17.50 | 2.40 | 0.90 | .013 |
| Mutagenic potency in YG1041-S9f (Rev./μg EOM) | 67.90 | 0.00 | 192.50 | 236.80 | 28.80 | 0.85 | .025 |
Mutagenicity potency values were obtained from Mutlu et al. (2013).
Data are slopes of linear regressions of dose-response data.
Sensitive to non-PAH base-substitution mutagens.
Sensitive to nitroarenes.
Sensitive to frameshift mutagens other than nitroarenes.
Sensitive to nitroarenes.
S9 is a supernatant fraction of a rat liver homogenate containing metabolizing enzymes; it is added to mimic mammalian metabolism of test substances since some mutagens require metabolism.
Rev., revertants.
Figure 5.
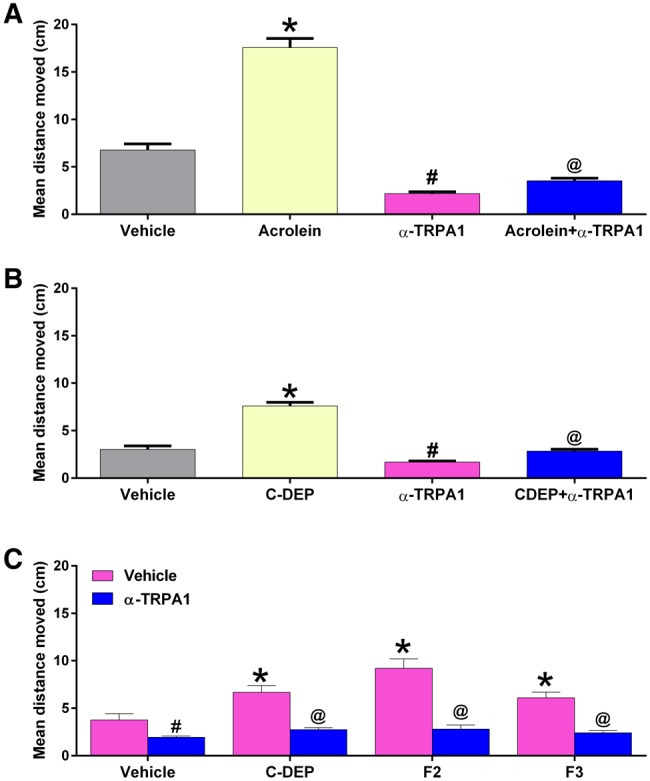
Effect of anti-TRPA1 pretreatment on zebrafish larval locomotor responses to acrolein (10 µM) (A), C-DEP (22 µg/ml) (B), and fractions F2 (10 µg/ml) and F3 (1 µg/ml) (C). Zebrafish were treated with 50 μM of the TRPA1 antagonist HC030031 beginning 15 min before treatment with each diesel extract. Data are presented as mean ± SEM for the entire 60-min period of recording. Data from 2 replicate plates were combined for a total of 24–48 zebrafish per exposure. vehicle = 0.8% DMSO. *Significantly greater than vehicle alone (P < .05). #Significantly less than vehicle alone. @Significantly less than corresponding chemical/extract-treated group without antagonist (P < .05).
RESULTS
Locomotor Responses to Negative and Positive Controls
The locomotor responses to 0.4% DMSO were not different from the responses to HBSS (Supplementary Figure 1A). Likewise, responses were not different among exposures to 0.4% or 0.8% DMSO (Supplementary Figure 1B), indicating that various vehicles do not perturb activity on their own.
Administration of acrolein caused an immediate statistically significant dose-dependent hyperactive locomotor response in zebrafish during the first 10 min of treatment (P < .05; Figure 2) with no effect of the lower concentrations (ie, 1 and 3 μM) during this period. However, during the remaining 50 min of treatment, locomotor activity in the zebrafish treated with 3 μM became significantly greater than control (P < .05), while zebrafish activity in the group treated with 30 μM was no longer significantly greater than control. In addition, zebrafish treated with 10 μM acrolein had significantly greater activity than control throughout the first 50 min after treatment (P < .05) but were not significantly different from control during the final 10 min of treatment. Interestingly, the locomotor activity in the zebrafish treated with 1 μM acrolein became significantly greater than control only during the last 20 min of monitoring (P < .05), ie, 40 min after the beginning of treatment.
Locomotor Responses to Whole C-DEP
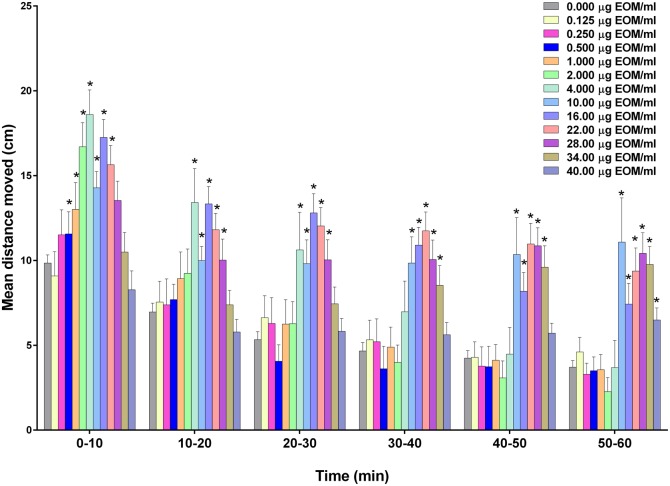
Treatment of zebrafish with C-DEP induced a concentration-dependent response that peaked soon after treatment (Figure 3), with significant increases in the 0.5–22 µg/ml treatment groups during the first 10 min of exposure (P < .05). Interestingly, there were no significant effects on locomotor activity with the 2 highest concentrations (34 and 40 µg/ml) during the first 30 min of exposure. During the final 30 min of exposure (P < .05), however, zebrafish treated with C-DEP at 34 µg/ml had significantly greater locomotor activity, whereas fish treated with 40 µg/ml had significantly greater locomotor activity only during the final 10 min of exposure (P < .05). C-DEP at 10, 16, 22, and 28 µg/ml induced hyperactivity during the entire 60 min of exposure. Locomotor behavior in response to acrolein and select C-DEP concentrations are shown in Supplementary Figures 1C and 1D to illustrate changes by 2-min increments.
Figure 3.
Effect of compressor-generated diesel exhaust particulate matter (C-DEP) on zebrafish larval locomotor activity measured in the dark. Zebrafish were exposed to 0.125–40 µg/ml C-DEP in 0.4% DMSO or 0.4% DMSO alone approximately 5 min before monitoring locomotor behavior. Data are presented as mean ± SEM for the entire 60 min of recording. The wide concentration-range necessitated the splitting of groups over 2 plates (0.0125–4 µg/ml on 1 plate and 10–40 g/ml on the second plate); each plate was done in duplicate. Data from 2 replicate plates were combined for a total of 15–40 zebrafish per exposure group. *Significantly greater than vehicle alone at corresponding time (P < .05).
Locomotor Responses to C-DEP Fractions
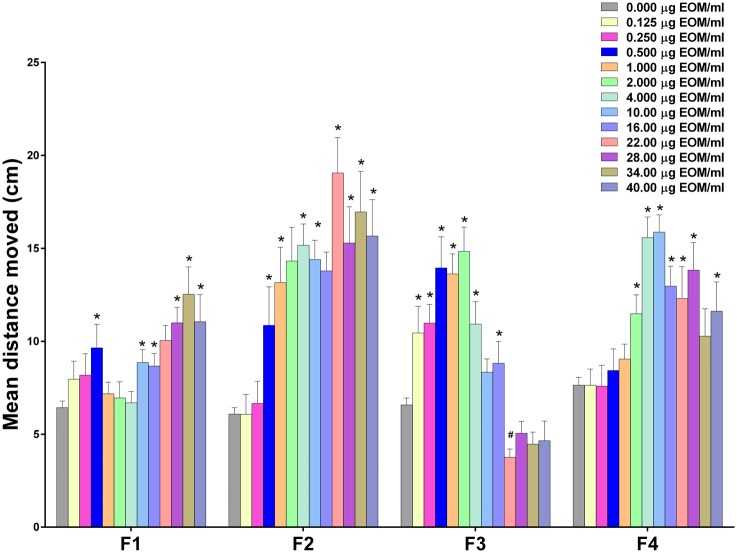
Treatment of zebrafish with each of the 4 fractions yielded concentration-dependent hyperactivity responses that varied considerably in activity levels (Figure 4). Zebrafish treated with F1, containing nonpolar constituents, yielded only mild responses that were largely restricted to the higher concentrations (10 μg/ml or greater) during the first 10 min of exposure (P < .05), with little evidence of hyperactivity after the first 10 min. Zebrafish treated with F2 in contrast, showed greater increases in activity at more doses, with significant increases in locomotor activity at concentrations as low as 0.5 μg/ml (P < .05) and extending through the highest concentration (ie, 40 μg/ml). Significantly increased activity was evident during the entire testing period for F2 concentrations ranging from 2 to 40 μg/ml (P < .05). Zebrafish treated with F3 were also very responsive as evidenced by significant increases in activity compared with control (P < .05) with the lowest concentration tested, ie, 0.125 μg/ml. Interestingly, however, zebrafish treated with F3 concentrations of 22 μg/ml and greater did not show increased activity at any point during treatment. Zebrafish treated with F4 had peak responses with 4 and 10 μg/ml, with significant effects occurring at concentrations as low as 1 μg/ml (P < .05). Locomotor activity in zebrafish treated with F4 concentrations at or above 4 μg/ml was significantly greater than the control group during the entire monitoring period (except for the first 10 min of activity in zebrafish treated with 34 μg/ml; P < .05).
Figure 4.
Effects of C-DEP fractions 1 (F1), 2 (F2), 3 (F3), and 4 (F4) on zebrafish larval locomotor activity measured in the dark during the first 10 min of recording. Zebrafish were exposed to 0.125–40 µg/ml of fractions 1–4 in 0.4% DMSO or 0.4% DMSO alone approximately 5 min before monitoring locomotor behavior. Data are presented as mean ± SEM. The wide concentration-range necessitated splitting the groups between 2 plates (0.0125–4 µg/ml on 1 plate and 10–40 µg/ml on the second plate), which were each done in duplicate. Data from 2 replicate plates were combined for a total of 15–32 zebrafish per treatment group. *Significantly greater than vehicle alone (P < .05). #Significantly less than vehicle alone (P < .05). Note: each fraction was run on separate plates with respective vehicle controls.
Linear regressions over the linear portion of the 0- to 10-min concentration-response data for C-DEP and all fractions (Table 2) showed that the potencies (ie, slopes; mean distance moved [cm]/µg EOM/ml) varied over 2 orders of magnitude (103-fold). Note: linear regressions were performed on data from the first 10 min because this was the most active period with all extracts. The linear range of each curve used to perform the regressions ranged from 0 µg EOM/ml to the highest concentration just before the curve plateaued for each dataset and one that yielded a regression with the highest goodness of fit (ie, r2 value). The linear ranges were as follows: C-DEP: 0–4 µg EOM/ml, F1: 0–22 µg EOM/ml, F2: 0–2 µg EOM/ml, F3: 0–0.5 µg EOM/ml, and F4: 0–4 µg EOM/ml. The extracts ranked as follows according to their irritant potencies (mean cm moved/µg EOM/ml): F3 (11.35) > F2 (4.96) > C-DEP (2.43) > F4 (1.79) > F1 (0.11). Thus, the fractions containing compounds that were weakly polar and polar (F2 and F3, respectively) elicited the most activity, whereas fractions at the extreme of polarity caused the least activity (F1 is nonpolar and F4 is highly polar).
Effects of TRPA1 Antagonism on Locomotor Responses to Acrolein and Extracts
We assessed the ability of a TRPA1 antagonist to block irritant responses to single concentrations of acrolein, C-DEP, and the 2 most potent fractions, F2 and F3 (we selected concentrations of acrolein and diesel extracts that elicited robust responses during the entirety of the monitoring periods in the concentration-response studies for the antagonism studies). Although the antagonist caused a significant reduction in locomotor activity relative to the DMSO control, it also caused a significant reduction in acrolein-induced locomotor responses relative to the DMSO control during the 60-min monitoring period (P < .05; Figure 5A). The antagonist also significantly reduced the locomotor responses induced by C-DEP and F2 and F3 (P < .05; Figs. 5B and 5C, respectively). Figure 5 shows the average values for the entire 60-min monitoring period.
DISCUSSION
Zebrafish treated with the positive control acrolein had both conventional and unconventional concentration-response patterns. Within the first 10 min of treatment, acrolein elicited a prototypical concentration-dependent increase in locomotor activity, consistent with previous studies (Prober et al., 2008). This immediate increase in activity was likely due to the irritant effects of acrolein at the superficial skin surface. Acrolein is a potent environmental pollutant with well-documented irritant effects in the respiratory tract (Bascom, 1991; Moretto et al., 2012; Morris et al., 1999). With time after treatment, however, the effects of higher doses diminished while that of lower doses increased. The nature of the mechanisms driving this divergence is unclear but may demonstrate the limits of this assay, wherein high doses elicit a lethargy-like state, perhaps due to overt toxicity or receptor downregulation from overstimulation.
The whole organic extract of C-DEP caused concentration-dependent hyperactive locomotor responses in zebrafish larvae that plateaued at intermediate concentrations. Except for effects during the latter part of the assay, C-DEP at higher concentrations, did not elicit hyperactivity, similar to the responses with high concentrations of acrolein and potentially owing to overt toxicity. These findings highlight the sensitivity of the assay and point to potential utility in discerning effects at PM concentrations in the ng range. These responses were immediate and consistent with well-documented PM-induced respiratory irritant responses in humans (Xu et al., 2013) and analogous responses in rodents (Farraj et al., 2011; Filep et al., 2016; Hemmilä et al., 2010; Sussan et al., 2014). Although responses decreased in magnitude by the end of the assessment, C-DEP’s effects remained elevated above controls, likely due to the continued presence of PM extract in the wells. The greater activity with high C-DEP concentrations late in the assay may have been due to a diminution of overt effects or a failure to habituate (a primitive form of learning and memory) because zebrafish treated with lower concentrations became hypoactive. Attenuation of habituation is an endpoint that has been noted in adult zebrafish treated with drugs (Wong et al., 2010). Given the similarity in responses to PM across species, and homologous sensory and inflammatory effects, zebrafish larval skin epithelial responses may be predictive of mammalian lung epithelial responses as previously proposed (McLeish et al., 2010).
C-DEP hyperactivity responses were likely driven by the presence of weakly polar and polar components as indicated by a comparison of responses to the fractions and whole C-DEP on an equal mass basis. F3, which was previously determined to have the highest concentration of polar nitro-PAHs and oxy-PAHs (Mutlu et al., 2013) of any of the fractions, caused the most activity in zebrafish. F2, which contained the highest concentration of weakly polar nitro-PAHs, was next most active. Similar locomotor responses were reported with exposure to PAHs found in oil-contaminated aquatic ecosystems (Vignet et al., 2014a,b). Oxy-PAHs have also been linked to developmental toxicity in zebrafish (Wincent et al., 2015). Furthermore, fine PM-induced increases in metabolizing enzymes (Olivares et al., 2013) and developmental effects (Mesquita et al., 2016) in zebrafish strongly correlated with PAH content. By contrast, the relative inactivity of F1 was likely due to a predominance of inert nonpolar unburned fuel components (ie, alkanes and alkenes). The reasons for reduced activity with F4, which contained highly polar compounds and some oxy-PAHs, are unclear and may relate to surface reactivity of such compounds and/or their capacity for penetration through hydrophobic zebrafish skin. Overall, these findings are striking in that weakly polar and polar components that account for only approximately 20% of the total EOM of C-DEP (approximately 52% and 28% of the mass eluted in F1 and F4, respectively) were responsible for most of the observed responses with C-DEP. Although PAH constituents are known drivers of PM-induced cytotoxicity and oxidative stress (Baulig et al., 2003; Li et al., 2002), non-PAH constituents (eg, quinones) may have contributed to the observed irritant responses given that PAHs were only a small fraction (0.3%) of the total EOM of a similar diesel particulate (Stevens et al., 2009).
Because the diesel-particle extracts used in this study have been previously evaluated for mutagenicity in strains of Salmonella that are sensitive to different chemical classes (Mutlu et al., 2013), we examined if the potencies of the extracts correlated between the Salmonella and zebrafish assays to provide further insight on the potential offending chemical classes that drove irritant responses in zebrafish. Among the 16 Salmonella strain/S9 combinations for which mutagenic potencies were determined (Mutlu et al., 2013), we found highly significant correlations between mutagenic potencies and fish irritant potencies for 4 strain/S9 combinations (Table 3). Two of these (TA98-S9 and YG1041-S9) respond to nitro-PAHs (nitroarenes), supporting the fact that F2 and F3, which are enriched in nitro-PAHs, were the most potent fractions in both assays. In addition, the mutagenic potencies of the extracts in strain TA100-S9 and TA98NR-S9, which detect base-substitution mutagens and frameshift mutagens that are not nitroarenes, respectively, also correlated with the irritant potencies of the extracts in the fish (Table 3). The precise identity of the chemicals that drove responses in zebrafish, however, require further study.
C-DEP hyperactivity responses were inhibited with an antagonist of TRPA1, a cation channel found on pain-sensing C-fiber neurons that is activated by chemical modification of N-terminal cysteinyl sulfhydryl groups (Takahashi and Mori, 2011). TRPA1 senses exogenous (eg, cinnamaldehyde) and endogenous (eg, inflammatory mediator 4-hydroxynonenal) chemicals (Takahashi and Mori, 2011). Importantly, DEP is pro-oxidant, and the same C-DEP used in this study activated proinflammatory signaling in human epithelial cells in an oxidative stress-dependent manner (Cao et al., 2007), providing a plausible mechanism for activation of TRPA1 in zebrafish given that zebrafish larvae express TRPA1 as early as 30-h postfertilization (Prober et al., 2008). Furthermore, whole diesel exhaust inhalation in rodents caused cardiac responses in part through TRPA1 activation (Hazari et al., 2011) and diesel particulates and diesel particulate PAHs activated TRPA1 in HEK293 cells transfected with human TRPA1 and in dorsal root ganglion neurons (Deering-Rice et al., 2011). Although other receptors and mechanisms (eg, inflammation) may play a role, the immediacy of the responses and the antagonism of effects suggest that C-DEP locomotor responses in zebrafish larvae were mediated by TRPA1.
Although behavior responses elicited after PM treatment were likely mediated by sensory pathways triggered in zebrafish skin, this study did not examine the role internalization of PM components played, particularly in the elicitation of responses recorded in the latter parts of the assay. Although there was no evidence of lethality, many organic components of PM, particularly hydrophobic chemicals such as PAHs, are absorbed by zebrafish (Kühnert et al., 2013), and once internalized are likely to trigger inflammatory responses (Bai and van Eeden, 2013). Future studies should include assessments of the amount of absorbed PM components in zebrafish and measures of inflammation. Similarly, although there were no differences in locomotor responses among various DMSO concentrations, the higher concentration of DMSO used in the antagonism studies may have influenced chemical uptake and should be examined in future studies.
Although this study examined the irritant potential of a diesel exhaust particulate, this zebrafish behavior assay is likely suitable for the assessment of other organic-rich PM sources and those dominated by water-soluble fractions containing metals. High sensitivity and reproducibility, correlation of results with other established assays, and assay simplicity make this model ideal for screening approaches requiring greater throughput. Taken together, zebrafish behavior assessments may help expedite toxicity determinations of PM sources, uncover mechanisms of action, and when coupled with chemical fractionation, identify causal chemical classes.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr Tamara Tal and Dr Bob Luebke of the U.S. Environmental Protection Agency, for their thorough review of this article, Dr Esra Mutlu, currently of the National Institute of Environmental Health Sciences in the United States, for extracting the diesel extracts, and John Havel of SRA International, Inc for producing the illustration in Figure 1.
FUNDING
This work was supported by the intramural research program of the Office of Research and Development (ORD), U.S. Environmental Protection Agency, Research Triangle Park, North Carolina, and included funding from ORD’s Pathfinder Innovation Projects.
REFERENCES
- Bai N., van Eeden S. F. (2013). Systemic and vascular effects of circulating diesel exhaust particulate matter. Inhal. Toxicol. 25, 725–734.http://dx.doi.org/10.3109/08958378.2013.844749 [DOI] [PubMed] [Google Scholar]
- Bascom R. (1991). The upper respiratory tract: Mucous membrane irritation. Environ. Health Perspect. 95, 39–44.http://dx.doi.org/10.1289/ehp.919539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulig A., Garlatti M., Bonvallot V., Marchand A., Barouki R., Marano F., Baeza-Squiban A. (2003). Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L671–L679. [DOI] [PubMed] [Google Scholar]
- Bessac B. F., Sivula M., von Hehn C. A., Escalera J., Cohn L., Jordt S. E. (2008). TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Invest. 118, 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R. D., Rajagopalan S., Pope C. A. I. I. I., Brook J. R., Bhatnagar A., Diez-Roux A. V., Holguin F., Hong Y., Luepker R. V., Mittleman M. A., et al. (2010). Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378. [DOI] [PubMed] [Google Scholar]
- Cao D., Tal T. L., Graves L. M., Gilmour I., Linak W., Reed W., Bromberg P. A., Samet J. M. (2007). Diesel exhaust particulate-induced activation of Stat3 requires activities of EGFR and Src in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L422–L429. [DOI] [PubMed] [Google Scholar]
- Claxton L. D., Creason J., Leroux B., Agurell E., Bagley S., Bryant D. W., Courtois Y. A., Douglas G., Clare C. B., Goto S., et al. (1992). Results of the IPCS collaborative study on complex mixtures. Mutat. Res. 276, 23–32. [DOI] [PubMed] [Google Scholar]
- Curtright A., Rosser M., Goh S., Keown B., Wagner E., Sharifi J., Raible D. W., Dhaka A., McKemy D. D. (2015). Modeling nociception in zebrafish: A way forward for unbiased analgesic discovery. PLoS One 10, e0116766.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering-Rice C. E., Romero E. G., Shapiro D., Hughen R. W., Light A. R., Yost G. S., Veranth J. M., Reilly C. A. (2011). Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): A probable mechanism of acute pulmonary toxicity for DEP. Chem. Res. Toxicol. 24, 950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino R., Staimer N., Tjoa T., Arhami M., Polidori A., Gillen D., Kleinman M., Schauer J., Sioutas C. (2010). Association of biomarkers of systemic inflammation with organic components and source tracers in quasi-ultrafine particles. Environ. Health Perspect. 118, 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farraj A. K., Hazari M. S., Haykal-Coates N., Lamb C., Winsett D. W., Ge Y., Ledbetter A. D., Carll A. P., Bruno M., Ghio A., et al. (2011). ST depression, arrhythmia, vagal dominance, and reduced cardiac micro-RNA in particulate-exposed rats. Am. J. Respir. Cell Mol. Biol. 44, 185–196. [DOI] [PubMed] [Google Scholar]
- Filep Á., Fodor G. H., Kun-Szabó F., Tiszlavicz L., Rázga Z., Bozsó G., Bozóki Z., Szabó G., Peták F. (2016). Exposure to urban PM1 in rats: Development of bronchial inflammation and airway hyperresponsiveness. Respir. Res. 17, 26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Wu M., Wang C., Wang Y., Zuo Z. (2015). Chronic exposure to low benzo[a]pyrene level causes neurodegenerative disease-like syndromes in zebrafish (Danio rerio). Aquatic Toxicol. 167, 200–208.http://dx.doi.org/10.1016/j.aquatox.2015.08.013 [DOI] [PubMed] [Google Scholar]
- Hazari M. S., Haykal-Coates N., Winsett D. W., Krantz Q. T., King C., Costa D. L., Farraj A. K. (2011). TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ. Health Perspect. 119, 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmilä M., Hihkiö M., Kasanen J. P., Turunen M., Järvelä M., Suhonen S., Pasanen A. L., Norppa H. (2010). Cytotoxicity and genotoxicity in vitro and irritation potency in vivo of two red phosphorus-based pyrotechnic smokes. Mutat. Res. 701, 137–144. [DOI] [PubMed] [Google Scholar]
- Hughes T. J., Lewtas J., Claxton L. D. (1997). Development of a standard reference material for diesel mutagenicity in the Salmonella plate incorporation assay. Mutat. Res. 391, 243–258.http://dx.doi.org/10.1016/S1383-5718(97)00075-2 [DOI] [PubMed] [Google Scholar]
- IARC. (2014). Diesel and Gasoline Engine Exhausts and Some Nitroarenes, Vol. 105. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France. [PMC free article] [PubMed]
- Jarema K. A., Hunter D. L., Shaffer R. M., Behl M., Padilla S. (2015). Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol. 52, 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus U., Breitner S., Schnelle-Kreis J., Cyrys J., Lanki T., Rückerl R., Schneider A., Brüske I., Gu J., Devlin R. (2011). Particle-associated organic compounds and symptoms in myocardial infarction survivors. Inhal. Toxicol. 23, 431–447. [DOI] [PubMed] [Google Scholar]
- Kühnert A., Vogs C., Altenburger R., Küster E. (2013). The internal concentration of organic substances in fish embryos–A toxicokinetic approach. Environ. Toxicol. Chem. 32, 1819–1827. [DOI] [PubMed] [Google Scholar]
- Li J., Ghio A. J., Cho S. H., Brinckerhoff C. E., Simon S. A., Liedtke W. (2009). Diesel exhaust particles activate the matrix-metalloproteinase-1 gene in human bronchial epithelia in a beta-arrestin-dependent manner via activation of RAS. Environ. Health Perspect. 117, 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Kim S., Wang M., Froines J., Sioutas C., Nel A. (2002). Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal. Toxicol. 14, 459–486.http://dx.doi.org/10.1080/089583701753678571 [DOI] [PubMed] [Google Scholar]
- Lim S. S., Vos T., Flaxman A. D., Danaei G., Shibuya K., Adair-Rohani H., Amann M., Anderson H. R., Andrews K. G., Aryee M., et al. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang Y., Zhu Z., Yang E., Feng X., Fu Z., Jin Y. (2016). Atrazine and its main metabolites alter the locomotor activity of larval zebrafish (Danio rerio). Chemosphere 148, 163–170. [DOI] [PubMed] [Google Scholar]
- MacPhail R. C., Brooks J., Hunter D. L., Padnos B., Irons T. D., Padilla S. (2009). Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 30, 52–58. [DOI] [PubMed] [Google Scholar]
- McLeish J. A., Chico T. J. A., Taylor H. B., Tucker C., Donaldson K., Brown S. B. (2010). Skin exposure to micro- and nano-particles can cause haemostasis in zebrafish larvae. Thromb. Haemost. 103, 797–807. [DOI] [PubMed] [Google Scholar]
- Mesquita S. R., Dachs J., van Drooge B. L., Castro-Jiménez J., Navarro-Martín L., Barata C., Vieira N., Guimarães L., Piña B. (2016). Toxicity assessment of atmospheric particulate matter in the Mediterranean and Black Seas open waters. Sci. Total Environ. 545–546, 163–170. [DOI] [PubMed] [Google Scholar]
- Moretto N., Volpi G., Pastore F., Facchinetti F. (2012). Acrolein effects in pulmonary cells: Relevance to chronic obstructive pulmonary disease. Ann. N. Y. Acad. Sci. 1259, 39–46. [DOI] [PubMed] [Google Scholar]
- Morris J. B., Stanek J., Gianutsos G. (1999). Sensory nerve-mediated immediate nasal responses to inspired acrolein. J. Appl. Physiol. 87, 1877–1886. [DOI] [PubMed] [Google Scholar]
- Mutlu E., Warren S. H., Matthews P. P., King C., Linak W. P., Kooter I. M., Schmid J. E., Ross J. A., Gilmour M. I., DeMarini D. M. (2013). Bioassay-directed fractionation and sub-fractionation for mutagenicity and chemical analysis of diesel exhaust particles. Environ. Mol. Mutagen. 54, 719–736. [DOI] [PubMed] [Google Scholar]
- Olivares A., van Drooge B. L., Casado M., Prats E., Serra M., van der Ven L. T., Kamstra J. H., Hamers T., Hermsen S., Grimalt J. O., et al. (2013). Developmental effects of aerosols and coal burning particles in zebrafish embryos. Environ. Pollut. 178, 72–79. [DOI] [PubMed] [Google Scholar]
- Oliveri A. N., Bailey J. M., Levin E. D. (2015). Developmental exposure to organophosphate flame retardants causes behavioral effects in larval and adult zebrafish. Neurotoxicol. Teratol. 52, 220–227.http://dx.doi.org/10.1016/j.ntt.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B., Feng W.-Y., Broadwin R., Green S., Lipsett M. (2007). The effects of components of fine particulate air pollution on mortality in California: Results from CALFINE. Environ. Health Perspect. 114, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla S., Hunter D. L., Padnos B., Frady S., MacPhail R. C. (2011). Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables. Neurotoxicol. Teratol. 33, 624–630. [DOI] [PubMed] [Google Scholar]
- Perrichon P., Le Menach K., Akcha F., Cachot J., Budzinski H., Bustamante P. (2016). Toxicity assessment of water-accommodated fractions from two different oils using a zebrafish (Danio rerio) embryo-larval bioassay with a multilevel approach. Sci. Total Environ. 568, 952–966. [DOI] [PubMed] [Google Scholar]
- Peterson R. T., MacRae C. A. (2012). Systematic approaches to toxicology in the zebrafish. Rev. Pharmacol. Toxicol. 52, 433–453.http://dx.doi.org/10.1146/annurev-pharmtox-010611-134751 [DOI] [PubMed] [Google Scholar]
- Prober D. A., Zimmerman S., Myers B. R., McDermott B. M. Jr, Kim S.-H., Caron S., Rihel J., Solnica-Krezel L., Julius D., Hudspeth A. J., et al. (2008). Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J. Neurosci. 28, 10102–10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Vera T., Pruneda-Álvarez L. G., Ochoa-Martínez A. C., Ramírez-GarcíaLuna J. L., Pierdant-Pérez M., Gordillo-Moscosoc A. A., Pérez-Vázqueza F. J., Pérez-Maldonado I. N. (2015). Assessment of vascular function in Mexican women exposed to polycyclic aromatic hydrocarbons from wood smoke. Environ. Toxicol. Pharmacol. 40, 423–429. [DOI] [PubMed] [Google Scholar]
- Shinyashiki M., Eiguren-Fernandez A., Schmitz D. A., Di Stefano E., Li N., Linak W. P., Cho S.-H., Froines J. R., Cho A. K. (2009). Electrophilic and redox properties of diesel exhaust particles. Environ. Res. 109, 239–244. [DOI] [PubMed] [Google Scholar]
- Stevens T., Cho S-H, Linak WP, Gilmour MI (2009). Differential potentiation of allergic lung disease in mice exposed to chemically distinct diesel samples. Toxicol. Sci. 107, 522–534. [DOI] [PubMed] [Google Scholar]
- Sun L., Xu W., Peng T., Chen H., Ren L., Tan H., Xiao D., Qian H., Fu Z. (2016). Developmental exposure of zebrafish larvae to organophosphate flame retardants causes neurotoxicity. Neurotoxicol. Teratol. 55, 16–22. [DOI] [PubMed] [Google Scholar]
- Sussan T. E., Ingole V., Kim J. H., McCormick S., Negherbon J., Fallica J., Akulian J., Yarmus L., Feller-Kopman D., Wills-Karp M., et al. (2014). Source of biomass cooking fuel determines pulmonary response to household air pollution. Am. J. Respir. Cell Mol. Biol. 50, 538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Mori Y. (2011). TRP channels as sensors and signal integrators of redox status changes. Front. Pharmacol. 2, 58.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson E. M., Breznan D., Karthikeyan S., MacKinnon-Roy C., Vuong N. Q., Dabek-Zlotorzynska E., Celo V., Charland J.-P., Kumarathasan P., Brook J. R., et al. (2016). Contrasting biological potency of particulate matter collected at sites impacted by distinct industrial sources. Part. Fibre Toxicol. 13, 65.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignet C., Devier M. H. L., Menach K., Lyphout L., Potier J., Cachot J., Budzinski H., Bégout M. L., Cousin X. (2014a). Long-term disruption of growth, reproduction, and behavior after embryonic exposure of zebrafish to PAH-spiked sediment. Environ. Sci. Pollut. Res. Int. 21, 13877–13887. [DOI] [PubMed] [Google Scholar]
- Vignet C., Le Menach K., Lyphout L., Guionnet T., Frère L., Leguay D., Budzinski H., Cousin X., Bégout M. L. (2014b). Chronic dietary exposure to pyrolytic and petrogenic mixtures of PAHs causes physiological disruption in zebrafish–Part II: Behavior. Environ. Sci. Pollut. Res. Int. 21, 13818–13832. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang X., Xiong C., Liu J., Hu B., Zheng L. (2015). Chronic bisphenol A exposure alters behaviors of zebrafish (Danio rerio). Environ. Pollut. 206, 275–281.http://dx.doi.org/10.1016/j.envpol.2015.07.015 [DOI] [PubMed] [Google Scholar]
- Wincent E., Jönsson M. E., Bottai M., Lundstedt S., Dreij K. (2015). Aryl hydrocarbon receptor activation and developmental toxicity in zebrafish in response to soil extracts containing unsubstituted and oxygenated PAHs. Environ. Sci. Technol. 49, 3869–3877. [DOI] [PubMed] [Google Scholar]
- Wittkopp S., Staimer N., Tjoa T., Stinchcombe T., Daher N., Schauer J. J., Shafer M. M., Sioutas C., Gillen D. L., Delfino R. J. (2016). Nrf2-related gene expression and exposure to traffic-related air pollution in elderly subjects with cardiovascular disease: An exploratory panel study. J. Expo. Sci. Environ. Epidemiol. 26, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K., Elegante M., Bartels B., Elkhayat S., Tien D., Roy S., Goodspeed J., Suciu C., Tan J., Grimes C., et al. (2010). Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav. Brain Res. 208, 450–457. [DOI] [PubMed] [Google Scholar]
- Wu S., Yang D., Pan L., Shan J., Li H., Wei H., Wang B., Huang J., Baccarelli A. A., Shima M., et al. (2016). Chemical constituents and sources of ambient particulate air pollution and biomarkers of endothelial function in a panel of healthy adults in Beijing, China. Sci. Total Environ. 560–561, 141–149. [DOI] [PubMed] [Google Scholar]
- Xia T., Korge P., Weiss J. N., Li N., Venkatesen M. I., Sioutas C., Nel A. (2004). Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: Implications for ultrafine particle toxicity. Environ. Health Perspect. 112, 1347–1358.http://dx.doi.org/10.1289/ehp.7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Barregard L., Nielsen J., Gudmundsson A., Wierzbicka A., Axmon A., Jönsson B. A., Kåredal M., Albin M. (2013). Effects of diesel exposure on lung function and inflammation biomarkers from airway and peripheral blood of healthy volunteers in a chamber study. Part. Fibre Toxicol. 10, 60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Staimer N., Tjoa T., Gillen D. L., Schauer J. J., Shafer M. M., Hasheminassab S., Pakbin P., Longhurst J., Sioutas C., et al. (2016). Associations between microvascular function and short-term exposure to traffic-related air pollution and particulate matter oxidative potential. Environ. Health 15, 81.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.