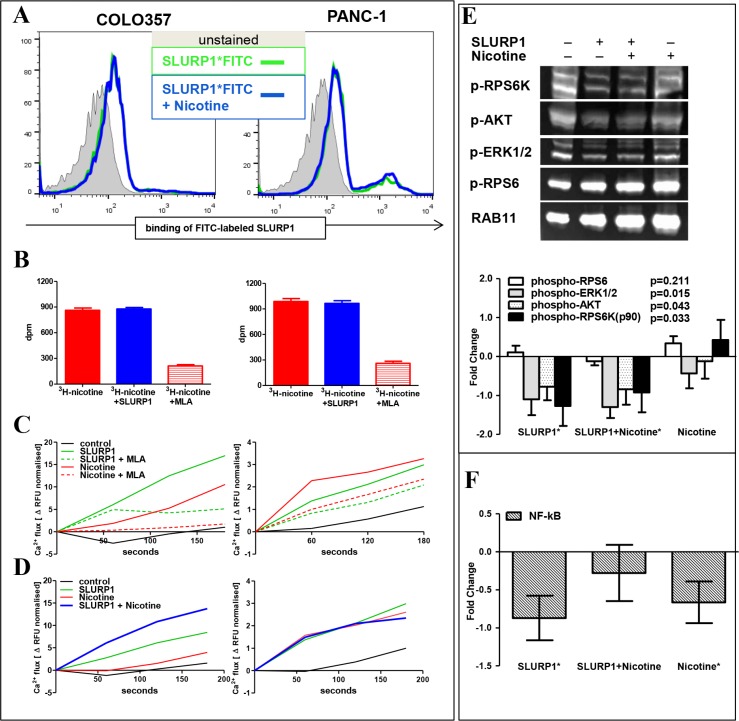
Figure 5. In PDAC cells, SLURP1 and nicotine occupy different binding sites and signal independently.
(A) FACS analysis of the PDAC cells showed no difference in binding of FITC-labeled SLURP1 in the absence (green line) or presence (blue line) of nicotine. (B) Radioligand assay showed no difference in binding of 3H-labeled nicotine in the absence (red bars) or presence (blue bars) of SLURP1, whereas exposure to a competitive CHRNA7-antagonist methyllycaconitine citrate (MLA) eliminated 80% of the binding. dpm: disintegrations per minute. (C) Both, SLURP1 and nicotine, induced MLA-sensitive Ca2+ influxes in COLO357 and PANC-1 cells. (D) Concurrent addition of SLURP1 and nicotine augmented Ca2+ influx in COLO357 (p = 0.01) and did no alter it in PANC-1 cultures. (E) Western blot analysis of the cellular lysates using the PathScan antibody cocktail showed that SLURP1 significantly inhibited basal phosphorylation of pAKT, pERK1/2, and p90RSK, but not of pRPS6 in COLO357, and this deactivation of potentially oncogenic signaling was maintained with concurrent exposure to nicotine. Nicotine alone showed no significant impact on the phosphorylation of these molecules. The lower panel summarizes data from four independent experiments and presents the quantification of the Western blot bands normalized to the RAB11 signal expressed as fold change over the untreated control. (F) TransAM-EIA assay, measuring the concentration of nuclear NF-κB in COLO357 cells, revealed that SLURP1 and nicotine significantly reduced the nuclear translocation of NF-κB when delivered alone but not together. The graphs summarize data from 5 to 14 independent experiments in duplicate or quadruplicate for each treatment. Significance level * = p < 0.05.