Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is associated with gestational diabetes mellitus (GDM). This study assessed the effects of continuous positive airway pressure (CPAP) in obese pregnant females with GDM and OSA.
Methods:
A randomized controlled trial was conducted (April 2014 – June 2016). Obese females at 24 to 34 weeks gestation and with diet-controlled GDM were screened for OSA. Those with OSA were randomly assigned to receive 2 weeks nightly CPAP or be part of a waitlist control group. After 2 weeks, all patients were offered CPAP. The primary outcome was glucose metabolism, obtained from an oral meal tolerance test (MTT) at baseline and 2 weeks. Pregnancy outcomes were collected.
Results:
Eighteen patients were randomized to CPAP and 18 to control groups. There were no significant changes between groups in fasting glucose, glucose response to MTT, and insulin sensitivity or secretion after 2 weeks. Those adherent to CPAP had significantly improved insulin secretion (P = .016) compared to the control group. When a counterfactual instrumental variable approach was applied to deal with nonadherence, the CPAP group had significantly improved insulin secretion (P = .002) and insulin sensitivity (P = .015). Lower rates of preterm delivery (P = .002), unplanned cesarean section (P = .005), and neonatal intensive care unit admissions (P < .001) were observed among those who used CPAP longer than 2 weeks.
Conclusions:
Two weeks of CPAP in females with GDM and OSA did not result in improved glucose levels, but insulin secretion improved in those adherent to CPAP. Continued CPAP use was possibly associated with improved pregnancy outcomes.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Title: Obstructive Sleep Apnea and Gestational Diabetes: Incidence and Effects of Continuous Positive Airway Pressure Treatment on Glucose Metabolism; Identifier: NCT02108197; URL: https://clinicaltrials.gov/ct2/show/NCT02108197
Citation:
Chirakalwasan N, Amnakkittikul S, Wanitcharoenkul E, Charoensri S, Saetung S, Chanprasertyothin S, Chailurkit L, Panburana P, Bumrungphuet S, Thakkinstian A, Reutrakul S. Continuous positive airway pressure therapy in gestational diabetes with obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2018;14(3):327–336.
Keywords: continuous positive airway pressure, gestational diabetes, glucose metabolism, obstructive sleep apnea, pregnancy outcomes
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) has been shown to increase risk of developing gestational diabetes mellitus (GDM). However, the data on the benefit of OSA treatment in GDM on glycemic control and fetomaternal outcome are currently lacking.
Study Impact: Obese pregnant females with diet-controlled GDM and who were adherent to continuous positive airway pressure (CPAP) had significantly improved β-cell function after 2 weeks. Continued use of CPAP was possibly associated with lower rates of preterm delivery, unplanned cesarean section, and neonatal intensive care unit admissions.
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway obstruction during sleep that results in oxygen desaturation or arousal.1 OSA is known to affect health consequences, particularly hypertension,2 coronary heart disease,3 stroke,4 and diabetes mellitus.5 The causal role of OSA in abnormal glucose metabolism has been documented through intermittent hypoxia and sleep fragmentation, leading to metabolism dysregulation.6,7 Emerging knowledge has also linked OSA to gestational diabetes mellitus (GDM). In a large population-based study of 3,132 females, the presence of OSA was associated with an increased risk for GDM (adjusted odds ratios 3.47 and 2.79 in early and mid-pregnancy, respectively).8 A meta-analysis of almost 10,000 pregnant females also demonstrated that coexisting OSA increased the odds ratio of developing GDM to 3.06.9 GDM is a significant health problem during pregnancy worldwide.10,11 In addition to being a risk for future type 2 diabetes, poorly controlled GDM is associated with serious perinatal complications.12
Despite the strong association between OSA and GDM, there is currently scarce information if treatment for OSA will result in improved glucose metabolism and possibly maternal-fetal outcomes. In nonpregnant populations, the results of continuous airway pressure (CPAP) treatment on glucose metabolism have been inconclusive, likely because of different patient populations and levels of adherence in the studies.13–18 Despite these data, the knowledge regarding the effects of CPAP on glucose metabolism in pregnancy is very much needed as glucose control in mothers can affect the fetal outcome. Therefore, this randomized controlled trial (RCT) was conducted to compare the efficacy of CPAP, a first-line treatment for OSA, to being a waitlist control on glucose metabolism in obese females with diet-controlled GDM and OSA.
METHODS
Participants
This RCT was conducted during April 2014 to June 2016. Adult pregnant females with clinically diagnosed GDM attending prenatal clinic at the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, were invited to undergo screening for OSA. Inclusion criteria for the screening step were diet-controlled GDM (diagnosed using The International Association of the Diabetes and Pregnancy Study Groups19), singleton pregnancy, gestational age (GA) between 24 and 34 weeks, and prepregnancy body mass index (BMI) ≥ 25 kg/m2 (obese per Asian criteria).20 We specifically enrolled females with diet-controlled GDM only because assessments of glucose metabolism, a primary outcome of this study, would have been more difficult in those requiring insulin with often ongoing medication adjustments. Participants in whom OSA was diagnosed were eligible for the RCT. Exclusion criteria were history of diabetes; sleep disorders; pulmonary, cardiac, and renal diseases; drug abuse; neurological/psychiatric disorders; use of medications affecting sleep or glucose metabolism; smoking; alcohol consumption > 7 drinks/wk; caffeine consumption > 400 mg/d; shift work; use of opioids/narcotics, alpha blockers, clonidine, methyldopa, or nitroglycerin.
All participants gave written informed consent. The protocol was approved by the Ethical Clearance Committee, Faculty of Medicine Ramathibodi Hospital (ClinicalTrials.gov NCT02108197).
Study Protocol
Baseline Characteristics, OSA and Glycemic Assessments
Age, current weight, and height were obtained. Symptoms of OSA were evaluated using two questionnaires, the Berlin Questionnaire21 and the Epworth Sleepiness Scale (ESS),22 both validated in Thai.23,24 The Berlin Questionnaire assesses three OSA risk categories: (1) snoring behavior, (2) wake time sleepiness or fatigue, and (3) BMI ≥ 30 kg/m2 and/or hypertension. The risk for OSA is considered high if significant symptoms exist in two of three categories. In addition, frequent snoring, a part of the Berlin Questionnaire category 1, was defined as present if the participants answered for the question “How often do you snore?” with “almost every day” or “3–4 times per week.”24 The ESS assesses daytime sleepiness symptoms, with higher scores reflecting more sleepiness.22 Self-reported sleep duration was derived from the question “During the past month, how many hours of actual sleep did you get at night on weekdays and weekends?” We then computed a weighted weekly average sleep duration using the following formula:
where SPD represents sleep duration.
OSA was diagnosed using a United States Food and Drug Administration-approved portable diagnostic device, Watch-PAT 200 (Itamar Medical, Ltd., Caesarea, Israel), which has been validated against polysomnography in pregnancy.25 The severity of OSA was assessed by a respiratory event index (REI). OSA was considered present if REI ≥ 5 events/h, mild if REI 5 to < 15 events/h, moderate if REI is 15–30 events/h, and severe if REI ≥ 30 events/h. Minimum O2 is lowest oxygen saturation value over the recording period. Because this device relies on peripheral arterial tone changes, use of certain medications as previously outlined was not allowed. The device cannot differentiate obstructive from central apnea events; however, central apnea is considered uncommon in pregnancy.26
Hemoglobin A1c and fasting plasma glucose (FPG) were obtain after an overnight fast, within 1 week of OSA assessment.
Randomization
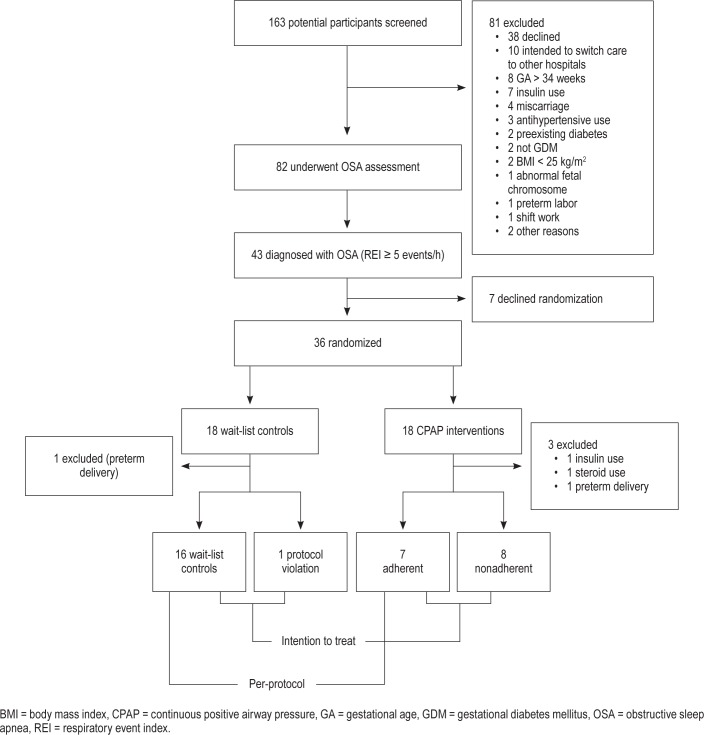
Of the 163 potential participants screened, a total of 82 females were eligible and underwent OSA assessment at a median GA of 29 weeks as shown in Figure 1. Of these, 43 (52.4%) had OSA, with a median REI of 9.4 (interquartile range 6.4, 12.4). Among those with OSA, 35 (81.3%) had mild, 7 (16.3%) had moderate, and 1 (2.3%) had severe OSA. The characteristics of the full cohort were previously reported in detail.27
Figure 1. Consort diagram.
Participants with OSA who agreed to participate in the study were randomly assigned to receive CPAP treatment or be a waitlist control for 2 weeks. A permuted block randomization with 1:1 ratio was used to generate randomization sequences. Before receiving treatment in their allocated arm, a mixed meal tolerance test (MTT) was performed after an overnight fast, using 250 cc of liquid meal (Isocal, 265 calories, 37% from fat, 50% carbohydrates and 13% protein). Glucose and insulin levels were obtained at 0, 30, 60, 90, and 120 minutes. MTT was previously used to assess glucose and insulin response in females with GDM.28 The participants randomized to CPAP were then instructed on CPAP use by the sleep specialist. After 2 weeks, an MTT was repeated. Participants in both groups received usual GDM care during this period.
Interventions
An auto-adjusting CPAP (APAP) machine was used during the study (S9 AutoSet with H5i humidifier, ResMed, San Diego, California, United States). They were set in the APAP mode with the setting of 4–20 cm H2O. The ramp time at the CPAP of 4 cm H2O was adjusted based on the participants' reported sleep latency. Humidifier was set at the temperature default of 25°C and humidification level default of 3, and further adjusted as needed. Nasal mask and nasal pillow were utilized during the study as appropriate. A follow-up phone call was conducted 1 day after the CPAP start and as needed thereafter. The downloaded data, including adherence, REI, and leak, were obtained at 2 weeks and monthly afterward until delivery if the participants wished to continue with the CPAP. Adherence to CPAP was defined as having used the device for ≥ 4 h/night and for ≥ 70% of nights.
Participants in the waitlist arm received their usual GDM care during the randomization period. All participants received diet counseling as part of GDM care in the obstetric clinic.
Metabolic Parameters and Outcome Measures
Primary outcomes were changes in glucose tolerance as measured by FPG and area under the curve (AUC) of glucose response (calculated using the trapezoid rule) to MTT before and after the 2-week randomization period. Secondary outcomes were changes in metabolic indices reflecting insulin secretion and insulin sensitivity as obtained from MTT. Homeostatic model assessment of insulin resistance (an index of fasting insulin resistance),29 the insulinogenic index (an estimate of early insulin secretion),30 and the Matsuda index (an index of whole body insulin sensitivity)31 were calculated. The disposition index, an indicator of β-cell function adjusted for insulin sensitivity, was calculated as a product of the insulinogenic index and the Matsuda index.32 These indices derived from an MTT were shown to have good correlation with those obtained from a 75-g oral glucose tolerance test33 and have been used in pregnancy.34 MTT was chosen over a standard oral glucose tolerance test because it would be inappropriate to give 75 g of glucose to females with GDM.
Postrandomization
At the end of the 2-week randomization period, all participants were offered CPAP treatment until the end of their pregnancy. This step was performed for ethical reasons. For those choosing CPAP treatment, they continued to meet with the sleep specialist every 4 weeks until delivery.
Pregnancy Outcomes
Although the study was not designed to detect differences in pregnancy outcomes, the following parameters were collected: rate of insulin use, total gestational weight gain, clinically diagnosed preeclampsia, GA at delivery, preterm delivery (fewer than 37 weeks), unplanned cesarean section (C-section), birth weight, Apgar score at 1 and 5 minutes, rate of small and large for gestational age (3rd and 97th percentile), and neonatal intensive care unit/special care nursery admissions of the newborns.
Statistical Analysis
Data are presented as mean ± standard deviation or median (interquartile range), and frequency (%). Independent t tests (or Mann Whitney U test where appropriate) and chi square test were used to analyze differences between groups for continuous and categorical data, respectively.
The statistical analysis for primary objective was performed based on intention to treat (ITT).35 This included all pregnant female patients who were primarily randomized to treatment, regardless of actual therapy. Two additional post hoc approaches (ie, per-protocol [PP], and counterfactual approaches) were performed.36 The PP analysis included only those who completed the randomly assigned interventions. The counterfactual approach by instrumental variable (IV) regression analysis assessed what outcome would have been (or potential outcome) for participants who did/did not comply with the assigned treatments.37,38
Linear regression analysis was applied to compare changes in metabolic parameters between CPAP and waitlist groups. Mean difference and 95% confidence interval (CI) were estimated.
For the counterfactual approach, IV analysis was applied considering the assigned intervention as the IV and the actual participants received intervention as the endogenous variable. The two-stage least-square regression models39,40 were applied to construct two equations as follows: First, the actual participants who received intervention were regressed on the assigned intervention. Second, changes in metabolic parameters were regressed on both actual received and assigned interventions. Covariables (age, BMI, REI, baseline fasting glucose levels [for the first equation]) were adjusted in the IV model. All analyses were performed using STATA version 14.0 (Stata-Corp LLC, College Station, Texas, United States). Values of P < .05 was considered as statistically significant.
Sample Size
The sample size was estimated based on testing two means of fasting glucose of 0.44 mmol/L (shown to be associated with macrosomia and C-section).41 A total of 36 participants were required based on type one error, power of test, and intervention per control ratio of 80%, 5%, and 1:1, respectively. Given an estimated prevalence of OSA in GDM about 50%,42 and CI width of 11%, a total of 80 pregnant females were required for screening.
RESULTS
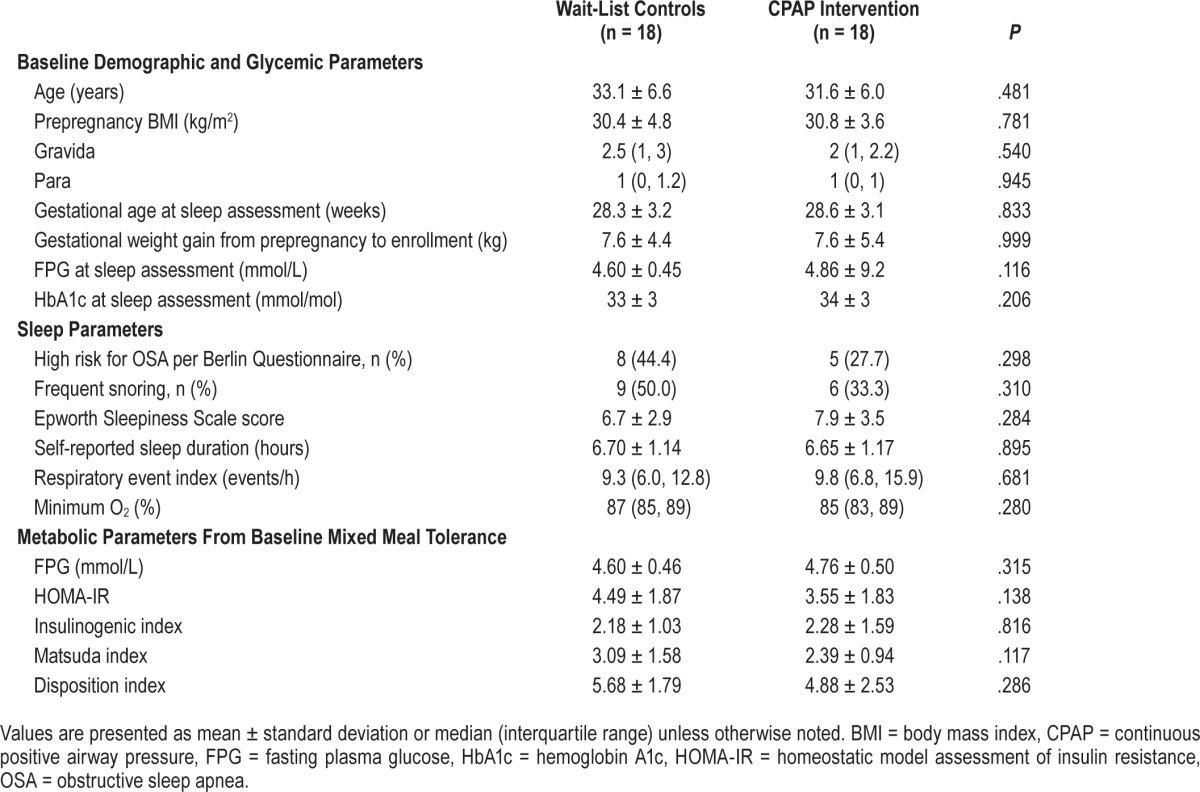
The consort diagram of the study is illustrated in Figure 1. Of the 43 participants with OSA, 7 declined randomization, resulting in 36 participants randomized to waitlist control and CPAP intervention groups (18 for each, see Figure 1 and Table 1). There were no significant differences between demographic, gestational weight gain, rates of being high risk for OSA or frequent snoring as evaluated by Berlin questionnaire, ESS score, self-reported sleep duration, REI, minimum O2, and baseline glycemic and metabolic parameters from MTT between the two groups (Table 1).
Table 1.
Demographic, glycemic, sleep, and metabolic parameters.
After randomization, three participants in the CPAP group (one received steroid, one received insulin, and one had pre-term delivery) and one in waitlist control arm (preterm delivery) were terminated, all before 2-week MTT assessment. This resulted in 15 and 17 participants in CPAP and waitlist control groups included in the ITT analysis, respectively.
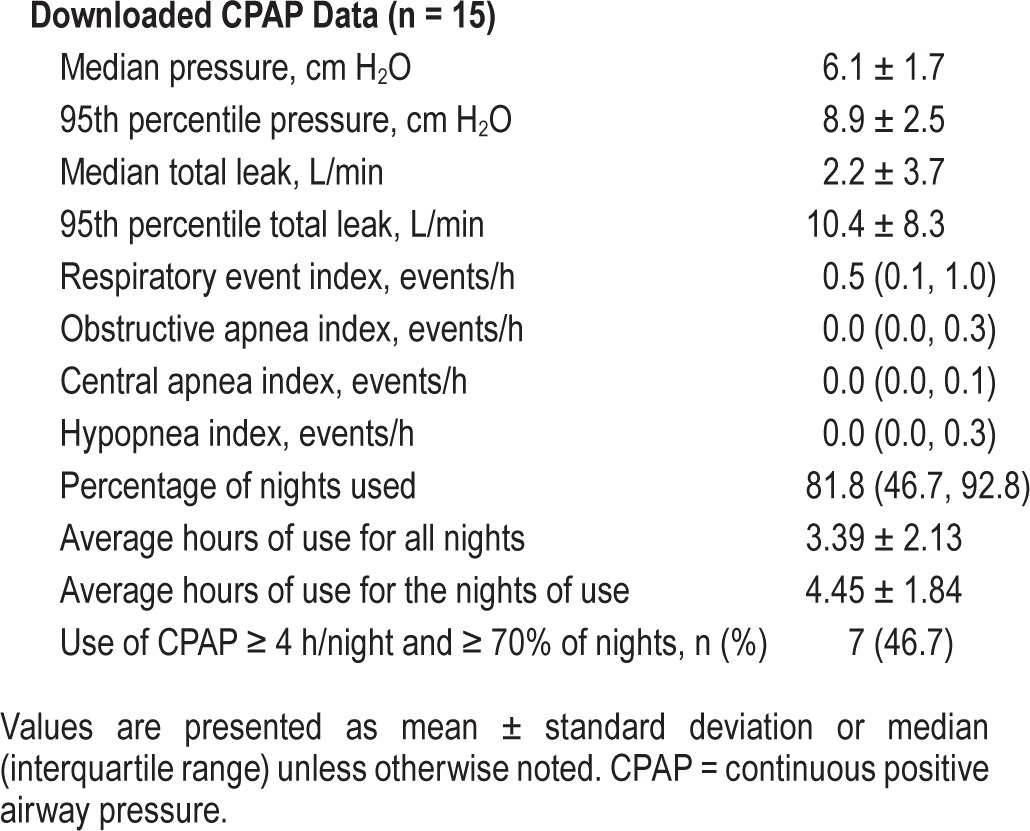
CPAP was started at an average GA of 30.3 weeks (SD = 2.9). The downloaded CPAP data revealed that 46.7% were adherent to CPAP (Table 2). The average hour ± standard deviation of usage on the days of use was 4.45 ± 1.84, and 3.39 ± 2.13 hours for all days. Median REI (interquartile range) was 0.5 (0.1, 1.0). REI was normalized (fewer than 5 events/h) in all except one participant in whom it was mildly elevated (7.8 events/h). However, the elevated REI observed in this patient was primarily an increase in central respiratory events (central apnea index of 7.5 events/h). Leak was acceptable in all participants.
Table 2.
Downloaded CPAP data after 2-week randomization.
The changes in metabolic parameters after randomization are shown in Table 3. As per ITT analysis, mean changes in fasting glucose were −0.17 and 0.12 mmol/L for CPAP and waitlist control arms respectively, with an estimated mean difference of −0.29 (95% CI: −0.62, 0.04). The mean changes of AUC glucose were not different between groups. No significant changes in other secondary outcomes were observed.
Table 3.
Change in metabolic parameters.
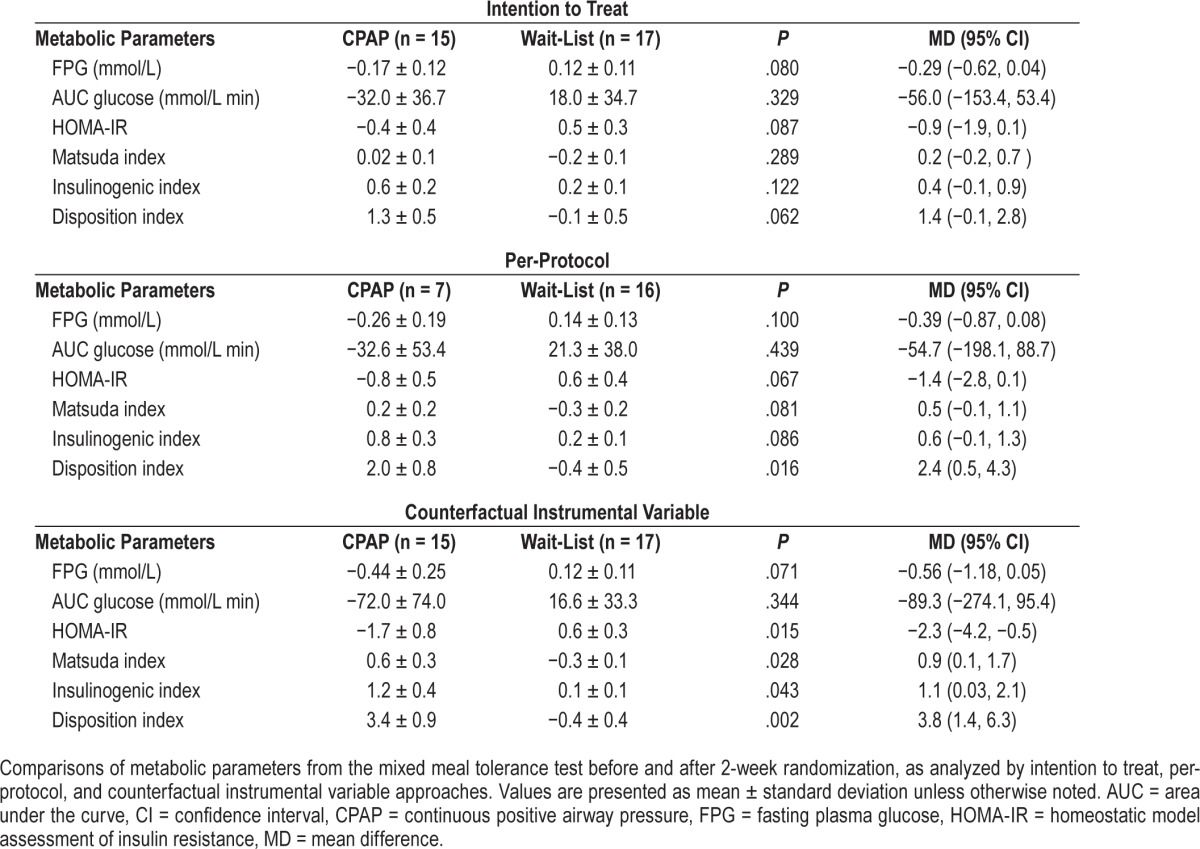
For PP analysis, eight and one participants in the CPAP and the waitlist control arms had protocol violations due to poor CPAP adherence and being inadvertently told to sleep on her side because of a positional apnea, respectively; leaving 7 and 16 participants for PP analysis. The mean difference in FPG was −0.39 (95% CI: −0.87, 0.08) mmol/L. Mean changes in AUC glucose were not significantly different between groups. However, the changes in the disposition index were significantly greater in the intervention group, with a mean difference of 2.4 (95% CI: 0.5, 4.3), P = .016, indicating improved β-cell function. Other secondary outcomes were not different between groups.
The IV analysis revealed a trend in decreased FPG in CPAP compared to waitlist control arms, mean difference −0.56 (95% CI: −1.18, 0.05) mmol/L, P = .07. In addition, the IV analyses could detect the effects of CPAP over the waitlist control arm on homeostatic model assessment of insulin resistance, Matsuda index, insulinogenic index, and disposition index with the mean differences of −2.3 (95% CI: −4.2, −0.5), 0.9 (95% CI: 0.1,1.7), 1.1 (95% CI: 0.03, 2.1), and 3.8 (95% CI: 1.4, 6.3), respectively (see Table 3). This suggested that CPAP would have resulted in improved insulin sensitivity and β-cell function if all the randomized participants had been CPAP adherent without any protocol violations.
Adverse Events During Randomization
There was one preterm delivery in the control group, and one preterm delivery with preeclampsia in the intervention group. One patient had a preterm contraction in the intervention group but carried her pregnancy to 37 weeks. Six patients had minor complaints (feeling uncomfortable with the mask, nasal congestion, and airflow that was too strong).
Postrandomization and Pregnancy Outcomes
Fourteen of 18 females in the intervention group chose to continue with CPAP and 10 in the waitlist control group started CPAP after the randomization period (Figure 2). There were no differences in maternal and fetal outcomes between intervention and waitlist control groups (Table 4).
Figure 2. CPAP use during postrandomization period.
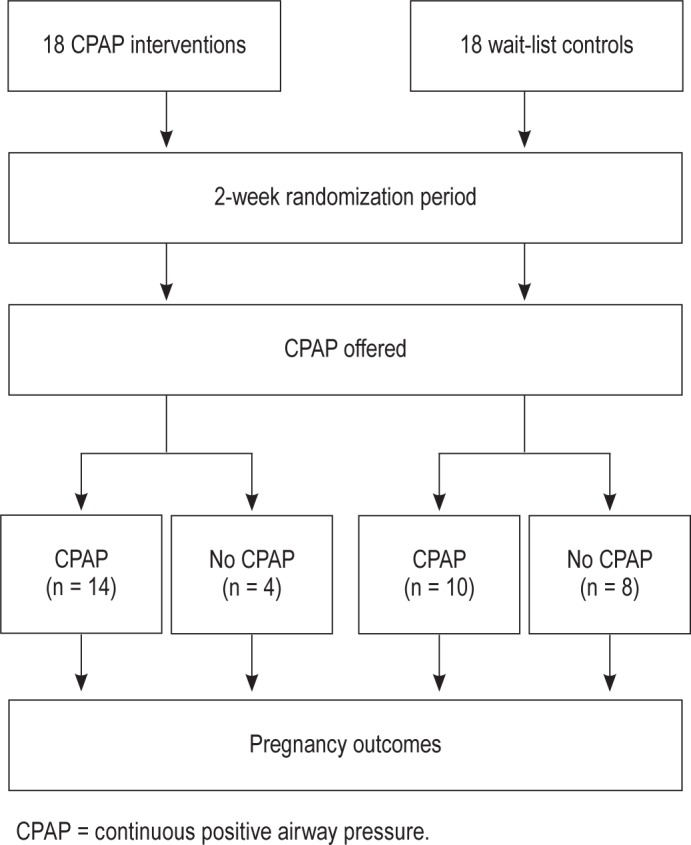
Table 4.
Pregnancy outcomes and data on CPAP use for the entire pregnancy period in randomized participants.
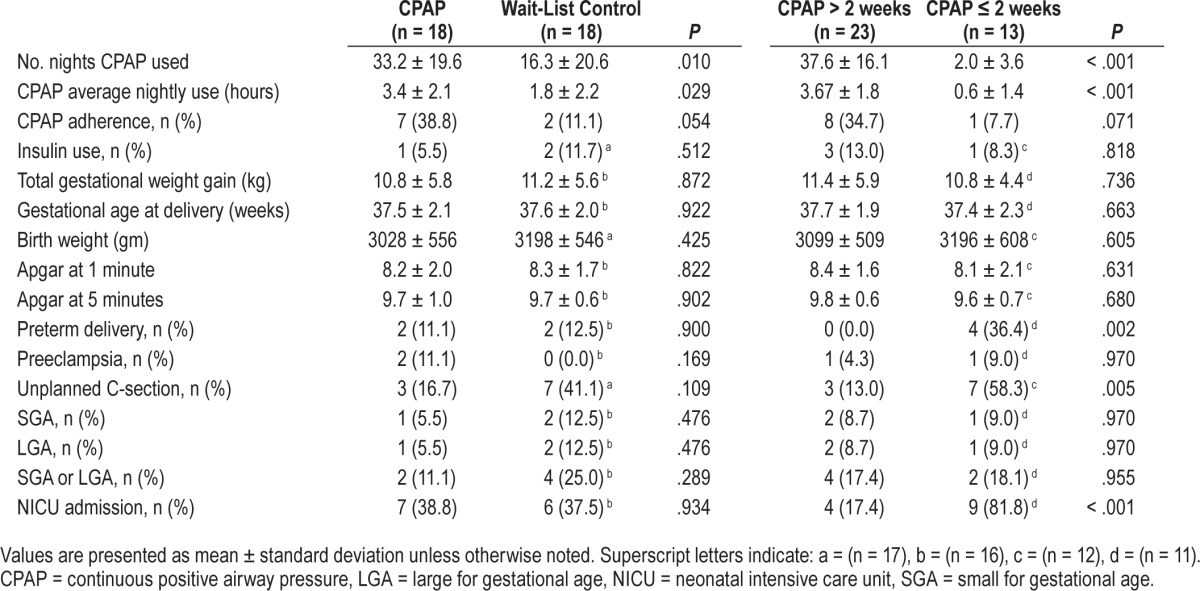
We further analyzed by grouping the patients according to those who used CPAP longer than 2 weeks (n = 23), and 2 weeks or less (including those who did not use CPAP at all, n = 13). This time period was chosen because it was the randomization duration, after which the patients could choose to terminate CPAP. The duration of CPAP use and adherence data is shown in Table 4. Compared to those using CPAP 2 weeks or less, those using CPAP longer than 2 weeks were less likely to have preterm delivery (zero versus 36.4%, P = .002), unplanned C-section (13% versus 58%, P = .005), and neonatal intensive care unit/special care nursery admissions (17.4% versus 81.8%, P < .001).
Characteristics Associated With Greater CPAP Use
We explored characteristics associated with greater CPAP use in the randomization and postrandomization period. During randomization, those who were adherent to CPAP (n = 7), compared to those who were nonadherent (n = 8), had more symptoms of daytime sleepiness at baseline (ESS: 9.6 ± 3.3 versus 6.0 ± 2.9, P = .045), whereas snoring symptoms and being at high risk for OSA per Berlin questionnaire did not differ between groups. REI was not significantly different between the two groups (adherent: 15.1 [7.2, 22.8] versus nonadherent: 9.3 [6.2, 10.1], P = .270).
During postrandomization, those who used CPAP for longer than 2 weeks also had more baseline daytime sleepiness compared to those who used the device 2 weeks or less (ESS: 8.1 ± 3.2 versus 5.4 ± 2.9, P = .05), whereas their Berlin and ESS scores were similar. REI was not statistically significant different between groups (CPAP > 2 weeks: 10.1 [7.2, 18.2] versus CPAP ≤ 2 weeks: 8.6 [5.6, 10.2], P = .118). There were no significant differences in other characteristics between the two groups, including age, prepregnancy BMI, GA at sleep assessment, gestational weight gain from prepregnancy until enrollment, FPG, or hemoglobin A1c levels.
DISCUSSION
In this study of obese, pregnant females with diet-controlled GDM and OSA, we demonstrated that 2 weeks of CPAP treatment in the third trimester was safe but did not result in improved glucose metabolism. However, there was a significant improvement in β-cell function as measured by a disposition index (by approximately 40%) in the participants who were adherent to CPAP. The counterfactual instrumental variable analysis demonstrated that CPAP treatment would have resulted in a significant reduction in fasting and whole-body insulin resistance, along with a significant improvement in β-cell function, with a trend in improving FPG levels, if all randomized participants had received interventions as specified in the protocol. These results further support the role of OSA in glucose metabolism in females with GDM. This treatment approach can be of benefit as better glycemic control is associated with better pregnancy outcomes.12
To our knowledge, our study is the first to explore the effects of CPAP treatment in pregnant females with GDM on metabolic parameters. The effect of CPAP on glycemic control in nonpregnant individuals with OSA and diabetes has had conflicting results.13–18 Variable CPAP adherence observed in these studies partly explained the inconsistent results. Furthermore, the overall pooled data demonstrated that the groups with more severe hyperglycemia and poorer glycemic control may be more beneficial with the use of CPAP compared to less severe and better glycemic control groups.13–18 In our participants, similarly to previous data in pregnant population, the OSA severity is generally mild.8 Hence, mild hyperglycemia, along with a relatively mild degree of OSA, possibly explained the insignificant change in overall glucose tolerance despite the improvement in β-cell function in participants who were adherent to CPAP in our study.
Intermittent hypoxia and sleep fragmentation are two main components of OSA. Each has been shown to adversely affect glucose metabolism. Although there has been no study in pregnancy, the data are available from a nonpregnant population. Five hours of exposure to intermittent hypoxia in healthy volunteers while awake resulted in a 17% reduction in insulin sensitivity and a 31% reduction in glucose effectiveness, without an increase in insulin secretion, suggesting a β-cell dysfunction.7 Intermittent hypoxia can also lead to increased sympathetic nervous system activity and proinflammatory cytokine secretion, leading to insulin resistance.43 In addition, an experimental sleep fragmentation resulted in a 25% decrease in insulin sensitivity.44 These mechanistic studies support the negative effect of OSA in glucose metabolism.
We also observed lower rates of preterm delivery, unplanned C-section, and infant admissions to neonatal intensive care unit in those who used CPAP longer than 2 weeks than those using CPAP 2 weeks or less. As OSA in pregnancy was reported to be associated with preterm delivery, C-section, and neonatal intensive care unit admissions,45 it is possible that there were some benefits in those who continued using CPAP despite not being fully adherent. Endothelial dysfunction, increased oxidative stress, and sympathetic nervous system overactivity were proposed as mechanisms linking OSA to adverse pregnancy outcomes.46 However, the data of pregnancy outcomes should be interpreted with caution because our study was not designed to capture these results and the number of the participants was relatively small.
Although our study has the strength of an RCT design, there are limitations. The randomization was not blinded because we did not use a sham CPAP. The CPAP adherence rate was relatively low, although similar to that of a general population (40% to 78%47) and previously published paper on the use of CPAP in those with OSA and diabetes, 2.5–7.9 h/night.26–31 This could be partly due to the relatively mild degree of OSA and OSA symptoms in the participants as reflected by their baseline ESS and Berlin Questionnaire results. This information, however, is in agreement with previous studies that suggested that questionnaire results were not accurate in predicting OSA in pregnancy.48–50 Although the number is relatively small and the results should be interpreted with caution, our data suggested that those with more symptoms of daytime sleepiness had greater CPAP use. This finding is supported by previous data in the nonpregnant population.51 Future research should explore factors related to barriers and adherence of CPAP use in pregnancy. Moreover, the intervention duration of 2 weeks is relatively short, although some data suggested the beneficial effects of CPAP on glucose metabolism as early as 1 week.52 There was also some concern that leaving pregnant females in the control arm untreated for more than 2 weeks may not be ethical. Other limitations include the lack of data of glucose home monitoring or a more detailed assessment of glucose levels such as a continuous glucose monitoring plus diet and exercise, although the weight gain during the 2-week randomization did not differ between groups (data not shown). As previously discussed, this study was performed with only diet-controlled obese participants with GDM and mild OSA. This degree of OSA might not have been treated in a general population. It remains to be investigated if the findings can be generalized to those treated with insulin, the less obese, or those with more severe OSA.
CONCLUSIONS
Two weeks of CPAP treatment in the third trimester of pregnancy in females with GDM was safe but did not result in significant changes in glucose levels. In those who were adherent to CPAP, significant improvement in β-cell function and a trend toward improvement in insulin sensitivity were observed. Continued CPAP use was possibly associated with improved pregnancy outcomes.
DISCLOSURE STATEMENT
Work for this study was performed at Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. This study was funded by The Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. The study equipment was supported by ResMed, Thailand. The funders had no role in study design or interpretations. Dr. Reutrakul reports grants from Merck Sharp and Dohme, nonfinancial support from ResMed, personal fees from Novo Nordisk, personal fees from Sanofi Aventis, personal fees from Medtronic, outside the submitted work. All other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Eve Van Cauter, Section of Adult and Pediatric Endocrinology, Diabetes, and Metabolism, and Sleep, Metabolism and Health Center, Department of Medicine, The University of Chicago, Chicago, Illinois, for her critical suggestions for the study. We also thank all the participants. Author contributions: N.C. researched and analyzed the data, wrote manuscript, contributed to discussion, reviewed/edited manuscript; S.A., E.W., S.C., S.S., S.Ch., L.C., P.P., and S.B. researched data, reviewed/ edit manuscript; A.T. analyzed data, reviewed and edited manuscript; S.R. conceptualized the study, researched and analyzed the data, wrote manuscript, contributed to discussion, reviewed/edited manuscript, and is the guarantor of this work and, as such, had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ABBREVIATIONS
- AUC
area under the curve
- BMI
body mass index
- C-section
cesarean-section
- CI
confidence interval
- CPAP
continuous positive airway pressure
- FPG
fasting plasma glucose
- GA
gestational age
- GDM
gestational diabetes mellitus
- HOMA-IR
homeostatic model assessment of insulin resistance
- ITT
intention to treat
- IV
instrumental variable
- LGA
large for gestational age
- MTT
meal tolerance test
- NICU
neonatal intensive care unit
- OGTT
oral glucose tolerance test
- OSA
obstructive sleep apnea
- PP
per protocol
- RCT
randomized controlled trial
- REI
respiratory event index
- SGA
small for gestational age
REFERENCE
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52(5):343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 6.Reutrakul S, Mokhlesi B. OSA and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106(5):1538–1544. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129(1):31–41. doi: 10.1097/AOG.0000000000001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luque-Fernandez MA, Bain PA, Gelaye B, Redline S, Williams MA. Sleep-disordered breathing and gestational diabetes mellitus: a meta-analysis of 9,795 participants enrolled in epidemiological observational studies. Diabetes Care. 2013;36(10):3353–3360. doi: 10.2337/dc13-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 11.Luengmettakul J, Sunsaneevithayakul P, Talungchit P. Pregnancy outcome in women with gestational diabetes mellitus according to the Carpenter-Coustan criteria in Thailand. J Obstet Gynaecol Res. 2015;41(9):1345–1351. doi: 10.1111/jog.12727. [DOI] [PubMed] [Google Scholar]
- 12.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 13.Myhill PC, Davis WA, Peters KE, Chubb SA, Hillman D, Davis TM. Effect of continuous positive airway pressure therapy on cardiovascular risk factors in patients with type 2 diabetes and obstructive sleep apnea. J Clin Endocrinol Metab. 2012;97(11):4212–4218. doi: 10.1210/jc.2012-2107. [DOI] [PubMed] [Google Scholar]
- 14.Shaw JE, Punjabi NM, Naughton MT, et al. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am J Respir Crit Care Med. 2016;194(4):486–492. doi: 10.1164/rccm.201511-2260OC. [DOI] [PubMed] [Google Scholar]
- 15.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62(11):969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mokhlesi B, Grimaldi D, Beccuti G, et al. Effect of one week of 8-hour nightly continuous positive airway pressure treatment of obstructive sleep apnea on glycemic control in type 2 diabetes: a proof-of-concept study. Am J Respir Crit Care Med. 2016;194(4):516–519. doi: 10.1164/rccm.201602-0396LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Ceron E, Barquiel B, Bezos AM, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(4):476–485. doi: 10.1164/rccm.201510-1942OC. [DOI] [PubMed] [Google Scholar]
- 18.Morariu EM, Chasens ER, Strollo PJ, Jr, Korytkowski M. Effect of continuous positive airway pressure (CPAP) on glycemic control and variability in type 2 diabetes. Sleep Breath. 2017;21(1):145–147. doi: 10.1007/s11325-016-1388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Western Pacific Region website. The Asia Pacific perspective: Redefining obesity and its treatment. [Accessed January 25, 2018]. http://www.wpro.who.int/nutrition/documents/Redefining_obesity/en/. Published February 2000.
- 21.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 23.Banhiran W, Assanasen P, Nopmaneejumruslers C, Metheetrairut C. Epworth sleepiness scale in obstructive sleep disordered breathing: the reliability and validity of the Thai version. Sleep Breath. 2011;15(3):571–577. doi: 10.1007/s11325-010-0405-9. [DOI] [PubMed] [Google Scholar]
- 24.Suksakorn S, Rattanaumpawan P, Banhiran W, Cherakul N, Chotinaiwattarakul W. Reliability and validity of a Thai version of the Berlin questionnaire in patients with sleep disordered breathing. J Med Assoc Thai. 2014;97(Suppl 3):S46–S56. [PubMed] [Google Scholar]
- 25.O'Brien LM, Bullough AS, Shelgikar AV, Chames MC, Armitage R, Chervin RD. Validation of Watch-PAT-200 against polysomnography during pregnancy. J Clin Sleep Med. 2012;8(3):287–294. doi: 10.5664/jcsm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourjeily G, Sharkey KM, Mazer J, Moore R, Martin S, Millman R. Central sleep apnea in pregnant women with sleep disordered breathing. Sleep Breath. 2015;19(3):835–840. doi: 10.1007/s11325-014-1099-1. [DOI] [PubMed] [Google Scholar]
- 27.Wanitcharoenkul E, Chirakalwasan N, Amnakkittikul S, et al. Obstructive sleep apnea and diet-controlled gestational diabetes. Sleep Med. 2017;39:101–107. doi: 10.1016/j.sleep.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Hollingsworth DR, Ney D, Stubblefield N, Fell T. Metabolic and therapeutic assessment of gestational diabetes by two-hour and twenty-four-hour isocaloric meal tolerance tests. Diabetes. 1985;34(Suppl 2):81–87. doi: 10.2337/diab.34.2.s81. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Seltzer HS, Allen EW, Herron AL, Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967;46(3):323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 32.Weiss R, Cali AM, Dziura J, Burgert TS, Tamborlane WV, Caprio S. Degree of obesity and glucose allostasis are major effectors of glucose tolerance dynamics in obese youth. Diabetes Care. 2007;30(7):1845–1850. doi: 10.2337/dc07-0325. [DOI] [PubMed] [Google Scholar]
- 33.Rijkelijkhuizen JM, Girman CJ, Mari A, et al. Classical and model-based estimates of beta-cell function during a mixed meal vs. an OGTT in a population-based cohort. Diabetes Res Clin Pract. 2009;83(2):280–288. doi: 10.1016/j.diabres.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Ekelund M, Shaat N, Almgren P, Groop L, Berntorp K. Prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetologia. 2010;53(3):452–457. doi: 10.1007/s00125-009-1621-3. [DOI] [PubMed] [Google Scholar]
- 35.Fisher LD, Dixon DO, Herson J, Frankowski RK, Hearron MS, Peace KE. Intention to Treat in Clinical Trials. In: Peace KE, editor. Statistical Issues in Drug Research and Development. 1st ed. New York, NY: Marcel Dekker, Inc; 1990. pp. 331–350. [Google Scholar]
- 36.McNamee R. Intention to treat, per protocol, as treated and instrumental variable estimators given non-compliance and effect heterogeneity. Stat Med. 2009;28(21):2639–2652. doi: 10.1002/sim.3636. [DOI] [PubMed] [Google Scholar]
- 37.Ye C, Beyene J, Browne G, Thabane L. Estimating treatment effects in randomised controlled trials with non-compliance: a simulation study. BMJ Open. 2014;4(6):e005362. doi: 10.1136/bmjopen-2014-005362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J. Determining causal exposure-response relationships with randomized concentration-controlled trials. J Biopharm Stat. 2014;24(4):874–892. doi: 10.1080/10543406.2014.901342. [DOI] [PubMed] [Google Scholar]
- 39.Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16(4):309–330. doi: 10.1177/0962280206077743. [DOI] [PubMed] [Google Scholar]
- 40.Staiger D, Stock JH. Instrumental variables with weak instrument. Econometrica. 1997;65:557–586. [Google Scholar]
- 41.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 42.Reutrakul S, Zaidi N, Wroblewski K, et al. Interactions between pregnancy, obstructive sleep apnea, and gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(10):4195–4202. doi: 10.1210/jc.2013-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 2010;24(5):843–851. doi: 10.1016/j.beem.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bin YS, Cistulli PA, Ford JB. Population-based study of sleep apnea in pregnancy and maternal and infant outcomes. J Clin Sleep Med. 2016;12(6):871–877. doi: 10.5664/jcsm.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cain MA, Louis JM. Sleep disordered breathing and adverse pregnancy outcomes. Clin Lab Med. 2016;36(2):435–446. doi: 10.1016/j.cll.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Russell T. Enhancing adherence to positive airway pressure therapy for sleep disordered breathing. Semin Respir Crit Care Med. 2014;35(5):604–612. doi: 10.1055/s-0034-1390070. [DOI] [PubMed] [Google Scholar]
- 48.Facco FL, Ouyang DW, Zee PC, Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med. 2012;8(4):389–394. doi: 10.5664/jcsm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson DL, Walker SP, Fung AM, O'Donoghue F, Barnes M, Howard M. Can we predict sleep-disordered breathing in pregnancy? The clinical utility of symptoms. J Sleep Res. 2013;22(6):670–678. doi: 10.1111/jsr.12063. [DOI] [PubMed] [Google Scholar]
- 50.Lockhart EM, Ben AA, Tuuli MG, Leighton BL. Obstructive sleep apnea in pregnancy: assessment of current screening tools. Obstet Gynecol. 2015;126(1):93–102. doi: 10.1097/AOG.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 51.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mokhlesi B, Grimaldi D, Beccuti G, Van Cauter E. Effect of one week of CPAP treatment of obstructive sleep apnoea on 24-hour profiles of glucose, insulin and counter-regulatory hormones in type 2 diabetes. Diabetes Obes Metab. 2017;19(3):452–456. doi: 10.1111/dom.12823. [DOI] [PubMed] [Google Scholar]