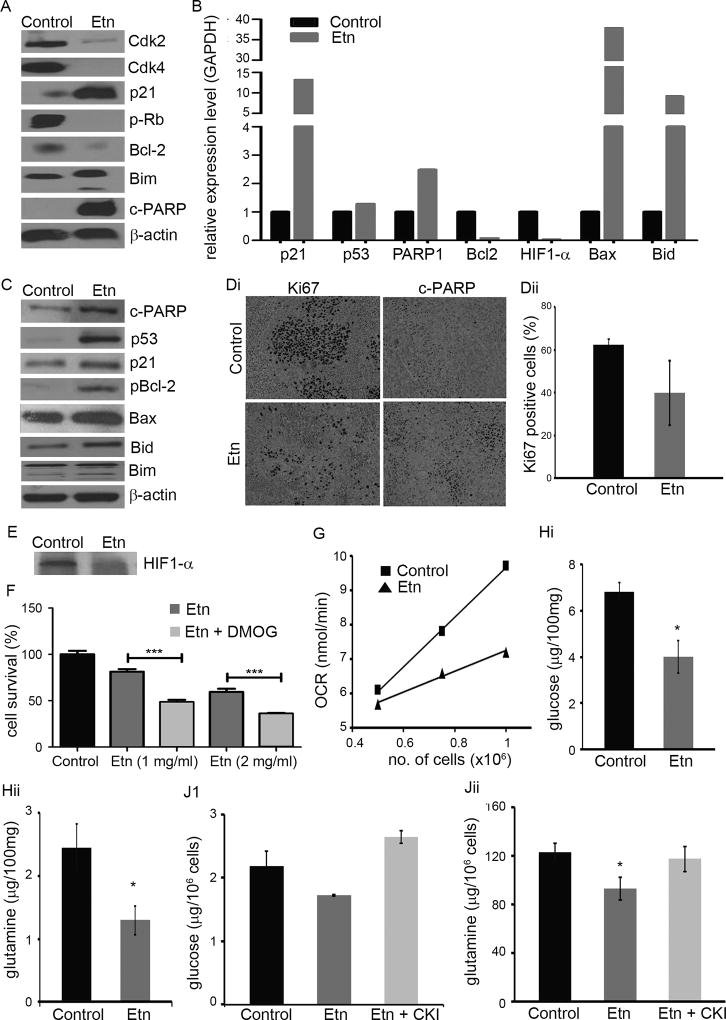
Figure 5.
Etn activates mitochondrially-mediated death pathways and affects oxygen consumption rate (OCR) and cellular metabolism in cancer cells. (A) Immunoblots of control and Etn-treated (2 mg/ml for 48h) PC-3 cell lysates for molecular regulators of cell-cycle (pRb, Cdk4, Cdk2, p21) and apoptosis (c-PARP, Bim, and Bcl-2). Beta actin was used as a loading control. (B) Relative transcripts of p21, p53, PARP1, Bcl-2, HIF1-α, Bax and Bid in control and Etn-treated (2 mg/ml for 48h) PC-3 cells. RNA samples were run on MOPS agarose gel to check integrity and two clear bands were observed for each sample. (C) Immunoblots of control and 40 mg/kg Etn-treated PC-3-luc tumors lysates for p53, p21, Bax, pBcl-2, c-PARP, Bim and Bid. Beta actin was used as a loading control. (Di) Micrographs showing immunohistochemical staining of Ki67 and c-PARP in control and Etn-treated PC-3-luc prostate xenografts. (Dii) Quantification of Ki67 staining in control and Etn-treated prostate xenografts. (E) Effect of Etn treatment on HIF1-α expression level in PC-3 luc prostate xenografts. (F) Effect of HIF1-α stabilization on Etn-induced cell death in PC-3 cells. PC-3 cells were pre-treated with 35µg/mL DMOG (HIF1-α activator) for 4h followed by treatment with 1 and 2 mg/ml Etn and DMOG together for 48h and estimation of cell survival by MTT assay. (G) Effect of Etn treatment on oxygen consumption rate in PC-3 cells. PC-3 cells were treated with 2 mg/ml Etn for 48h at pH 7.4 and cells were suspended at a concentration of 0.5, 0.75 and 1 million/ml. OCR was measured by using oxygen electrode. Measurements were initiated by adding 500 µl of control and 2 mg/ml Etn-treated cell suspension at various concentrations into electrode chamber pre-equilibrated with 500 µl fresh media. The plot shows representative OCR as a function of cell number for control and Etn-treated cells. Intracellular glucose (Hi) and glutamine (Hii) levels in control and 40 mg/kg Etn-treated PC-3-luc tumors. Glucose and glutamine levels in control and Etn-treated tumors were estimated by LC-MS/MS method. (J) Effect of choline kinase inhibition on intracellular levels of glucose and glutamine in Etn-treated cells. Treatment of PC-3 cells with 2 mg/ml Etn for 48h reduced intracellular level of glucose (Ji) and glutamine (Jii) and this reduction in glucose and glutamine level was abrogated upon inhibition of choline kinase enzyme. Values and error bars shown in the figure represent mean and SE respectively from three independent experiments (*, p < 0.05 compared with control; ***, p < 0.0001 compared with Etn treatment).