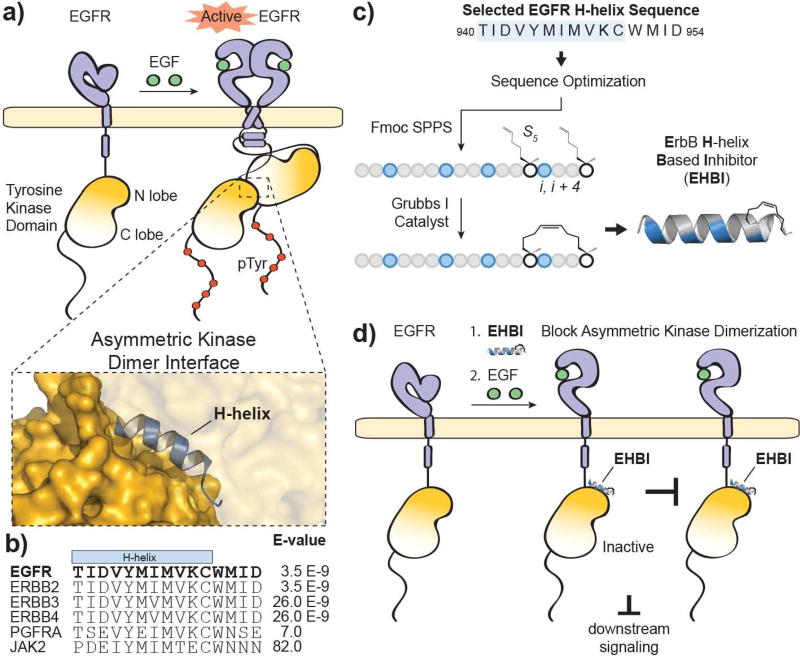
Figure 1. Targeting disruption of EGFR activation.
a) Ligand-dependent activation of EGFR results in dimerization through multiple interfaces including the extracellular ligand-binding domain, intracellular juxtamembrane segments and intracellular tyrosine kinase domains. The kinase domains form an asymmetric dimer where one tyrosine kinase domain (“activator”) allosterically activates the partner tyrosine kinase domain (“receiver”). The structure was rendered using PyMOL X11 (PDB 2GS6). b) An alignment of the top hits produced from a BLASTP search of the EGFR protein sequence surrounding the H-helix (residues 940–954; accession number P00533). c) A scheme for the design and synthesis of stapled ErbB H-helix Based Inhibitor (EHBI) peptides. d) A peptide mimic of the EGFR H-helix was designed as a strategy to block asymmetric kinase dimerization and inhibit EGFR activation.