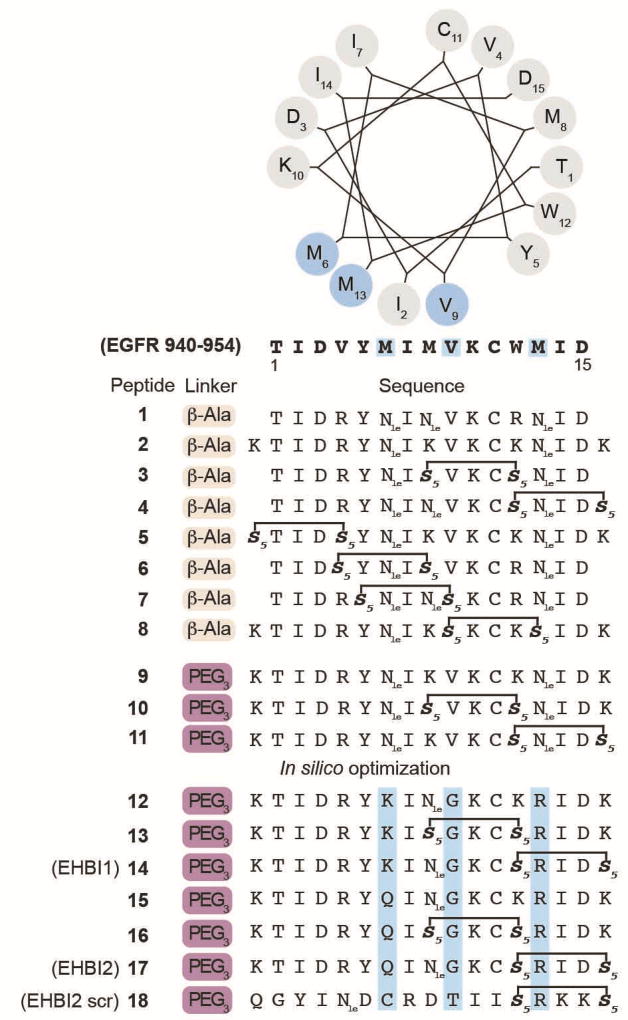
Figure 2. H-helix-derived peptide sequences.
The selected EGFR protein sequence (residues 940–954, accession number P00533) is presented as a helical wheel numbered as residues 1–15 (N- to C-terminus). Highlighted in light blue are residues that interact with the EGFR kinase domain (PDB 2GS6). In silico designed peptides (12–18) contain amino acid substitutions that were predicted to have relatively favorable target binding as compared to the wild-type sequence. Additional amino acid substitutions (K) were introduced to the anticipated solvent face of the peptide to improve solubility. The helical wheel was generated from the following site: http://kael.org/helical.htm. Nle = norleucine.