Abstract
Purpose
Central visual field (VF) damage in glaucoma patients can significantly hinder daily activities. The present study investigates whether the presence of localized baseline damage to the central ten degrees of the VF is predictive of faster global mean deviation (MD) progression.
Design
Prospective, cohort study.
Methods
Eyes from the multicenter African Descent and Glaucoma Evaluation Study (ADAGES) with established glaucoma and VF loss and a minimum of five 24-2 VFs were eligible. Baseline central 24-2 damage was defined as any of the 12 central-most points with total deviation (TD) values at P<0.5% on two consecutive examinations. Progression was determined using trend-based and event-based criteria: (i) rates of MD change significantly faster than zero and (ii) >−5 dB MD loss over the entire follow-up.
Results
827 eyes of 584 patients were studied. Mean rate of MD change of the entire sample was −0.15 dB/year (95% CI: −0.19 to −0.12, P<0.001). Eyes with baseline central damage progressed faster than those without (difference: βcentral = −0.07 dB/year, 95% CI=−0.11 to −0.01, P=0.011) and were more likely to experience MD loss greater than 5 dB [hazard ratio=3.0 (95% CI: 2.1 to 4.1, P<0.001)]. These differences remained significant after adjusting for confounders.
Conclusion
The presence of central VF damage at baseline is significantly associated with more rapid global progression. Detection of central VF damage aids in stratification of high-risk patients who may need intensive surveillance and aggressive treatment.
Keywords: central visual field damage, risk factors, glaucoma, visual fields, progression, nasal step, mean deviation, mean deviation sensitivity, visual field index
INTRODUCTION
The prevalence of glaucoma, the leading worldwide cause of irreversible blindness, will increase to 111.8 million individuals by 2040.1 Developing strategies to effectively screen and identify individuals at greater risk for more rapid glaucoma progression will be imperative to curtailing the effects of the disease.
Patterns of visual field (VF) loss and their effect on the progression of disease are an area of interest in glaucoma research.2–4 The central VF is regarded as an important area for glaucoma monitoring, as loss in this region has been associated with a greater degree of morbidity5, 6 and longitudinal decline in quality of life than more peripheral loss.7–9 Moreover, there have been reports that central VF loss can cause significant hindrances on daily activities such as reading10 and driving,11 so that monitoring central VF loss may be particularly important for patient well-being.
While it has been established that the central VF is an important determinant of vision-related quality of life, the impact of baseline damage in this area on future VF progression has not been fully investigated. The present study aims to investigate the impact of baseline damage to the central field on global VF progression. We hypothesize that baseline damage within the central 10 degrees of the 24-2 VF strategy is predictive of faster mean deviation (MD) rates of progression.
METHODS
Data for this analysis was collected as part of the multi-site, prospective, cohort study, African Descent and Glaucoma Evaluation Study (ADAGES). This collaboration (clinicaltrials.gov Identifier: NCT00221923) includes the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California-San Diego (UCSD) (data coordinating center), Edward S. Harkness Eye Institute at Columbia University Medical Center (site formerly located at New York Eye and Ear Infirmary), and the Department of Ophthalmology, University of Alabama-Birmingham (UAB). The institutional review boards at all sites approved the study methodology, which adheres to the tenets of the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act. All participants gave written informed consent. ADAGES enrollment began in January 2003 and ended in July 2006, and follow-up continued into 2016. Participants of both African and European ancestry were included in this longitudinal cohort study of individuals with healthy eyes, glaucoma suspects, and established glaucoma.
Participants
Participants were asked to identify their race by self-report using the National Eye Institute inclusion/enrollment system describing ethnicity and race (http://orwh.od.nih.gov/pubs/outreach.pdf [pages 120–121]). Information regarding a family history of glaucoma (biological mother, father, sibling, aunt, uncle, and grandparent) was also obtained. All participants were recruited from the glaucoma clinics and ophthalmic practices at each of the three recruiting sites, by advertisement and community presentations, and by referral from other ophthalmologists and optometrists in the community.
The ocular testing completed for ADAGES has been described elsewhere.12 In brief, participants underwent a comprehensive ophthalmic examination, including annual review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP), dilated funduscopic examination, pachymetry, simultaneous stereoscopic optic disc photography, and standard automated perimetry visual field (VF) testing with 24-2 Swedish interactive threshold algorithm (Carl Zeiss Meditec, Inc., Dublin, California, USA). VFs were repeated every 6 months and optic disc photographs were performed every 12 months.
Inclusion criteria at baseline
All participants had open angles, a best-corrected visual acuity ≥ 20/40, and a refractive error <5.0 diopters sphere and <3.0 diopters cylinder. At least one high-quality stereophotograph and two reliable (<33% false positives, false negatives, and fixation losses) standard automated perimetry Humphrey 24-2 field test results at baseline were required. Both eyes were included, except in cases where only one eye met the study criteria. Diabetic participants without evidence of retinopathy were included. Each participant underwent VF testing using the 24-2 program on the Humphrey Field Analyzer II, with the Swedish Interactive Thresholding Algorithm (SITA), 33 version 4.1 (Carl Zeiss Meditec, Inc., Dublin, California, USA). Only patients with glaucomatous optic neuropathy and abnormal 24-2 VFs (as defined below) were included in the present report.
Exclusion criteria
Participants were excluded if they had a history of intraocular surgery (except for uncomplicated cataract surgery or glaucoma surgery), secondary causes of glaucoma (e.g., iridocyclitis, trauma), other systemic or ocular diseases known to affect the VF (e.g., pituitary lesions, demyelinating diseases, etc.), significant cognitive impairment, history of stroke, Alzheimer disease, or dementia, problems other than glaucoma affecting color vision, an inability to perform VF examinations reliably, or a life-threatening disease that precluded retention in the study.
Glaucomatous Optic Neuropathy
Details of the ADAGES methodology have been described elsewhere.12 Glaucomatous optic neuropathy was defined as excavation, neuroretinal rim thinning or notching, localized or diffuse retinal nerve fiber layer defect, or vertical cup-disc ratio asymmetry > 0.2 between eyes (not explained by differences in disc size) based on masked grading of stereophotographs by two graders at the Imaging Data Evaluation and Analysis (IDEA) Reading Center. Only photographs of adequate quality were used for evaluation.
An abnormal 24-2 VF was defined as pattern standard deviation (PSD) had a P<5% or the Glaucoma Hemifield Test (GHT) result was “outside normal limits.” Abnormality had to be confirmed with an additional VF test.12
Subjects with glaucomatous optic neuropathy, abnormal 24-2 VFs, and at least five 24-2 VFs were included in this study.
Visual Field Progression
Progression was defined using: (i) trend analysis: significant rate of MD change (significantly different from zero) using mixed effects linear models and (ii) event analysis: >5 dB MD loss over the entire follow-up period. This cut-off value was chosen as it has been reported that MD losses >5 dB are associated with significant decline in vision-related quality of life.13
Since the 24-2 MD calculation allots greater weight to the central points of the VF, which could potentially overestimate the effect of baseline central damage, we also analyzed models with mean sensitivity deviation (MSD) as the outcome variable. The MSD is calculated after simple averaging of the deviation sensitivities from the TD plots. Of note, given the that these values are in log scale (dB), they were first converted to linear scale, averaged, and then converted back to dB. Additionally, we modeled Visual Field Index (VFI) as the outcome variable. Since VFI gives the central field even more weight than MD, we hypothesized that any potential association between central field loss and global progression would be most strongly demonstrated with this measure.
Central VF damage and Nasal VF Damage
Central 24-2 damage was defined as any of the 12 central-most points with total deviation (TD) values categorized as abnormal at P<0.5% on two consecutive baseline examinations.
One potential limitation of the proposed analysis is that patients with baseline central VF damage are likely to have a worse overall VF status due to the presence of a greater number of abnormal test locations (P<0.5%). Therefore, we completed an additional analysis in which we evaluated the predictive value of baseline nasal VF damage using GHT sector 3, which consists of 10 points on VF progression. We chose this sector as nasal VF damage is often one of the earliest sites of glaucomatous functional damage.14–16 We tested whether the presence of any significantly abnormal test location (as defined by TD probabilities at P<0.5%) in the nasal sector and central visual field was associated with VF progression. Figure 1 demonstrates the central and nasal points studied in this report.
FIGURE 1.
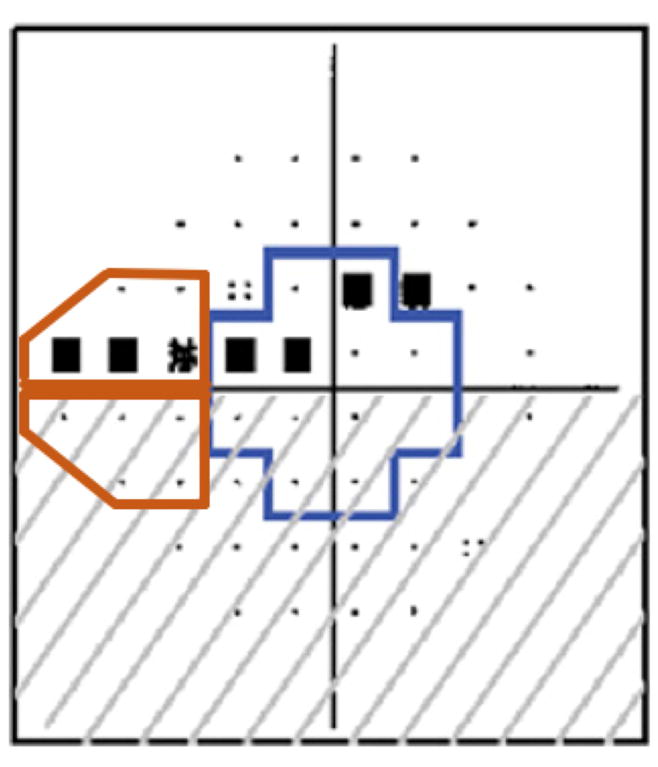
The central visual field was defined as the 12 points outlined in blue on the 24-2 field, while the nasal visual field was defined as the 10 points outlined in orange on the 24-2 field.
Statistical analysis
Measures of center and dispersion are described as means and standard deviation (SD), respectively. Categorical variables were compared using Fisher’s exact-test and continuous variables were compared using two-tailed, independent samples t-test. Linear mixed-effects models were used to evaluate rates of change in VF MD over time and their relationship with the presence of baseline central VF damage (as defined above). In linear mixed models, the average evolution of the outcome variable (MD) is described using a linear function of time, and random intercepts and random slopes introduce subject- and eye-specific deviations from this average evolution. The model accounts for the fact that two eyes from one individual can have different rates of MD loss over time, while also correcting for correlations between two eyes from one individual. Cox proportional hazards models were used to test the relationship between predictors and the event-based outcome.
The following potential confounders were included as covariates in the model: baseline VF MD, age at baseline, central corneal thickness, and mean follow-up IOP. The two-way interaction between “Time” and “Presence of baseline central VF defect” was included in the model in order to evaluate whether there was a significant difference in MD slopes (dB/year) between eyes with and without baseline central VF damage. Additionally, we included the two-way interaction terms for all covariates, particularly the baseline MD, to minimize the effect of a worse baseline severity in patients with central defects on the rates of MD change.
Statistical analyses were performed using commercially available software (STATA, version 14; StataCorp LP, College Station, TX). The α level (type I error) was set at 0.05.
RESULTS
Eight hundred and twenty-seven eyes of 584 patients met our inclusion criteria and are included in this report. Mean (standard deviation, SD) age at baseline was 63 (12) years for those with baseline central damage and 60 (12) years for those without (P<0.001). Eyes with baseline central loss had a mean of 13.7 (5) VFs spanning 8.8 (3) years, while eyes without baseline central loss had a mean of 13.7 (6) VFs spanning 8.8 (4) years (P>0.05). As expected, baseline MD was worse in those eyes with baseline central damage compared to those without (−9.0 (6) dB vs. −2.2 (2) dB, P<0.001). Additionally, baseline MSD was worse in those with baseline central damage compared to those without (−9.0 (6) dB vs. −2.5 (2) dB, P<0.001). Patient characteristics are summarized in Table 1.
TABLE 1.
Characteristics of the study sample by central visual field damage. Data shown as mean (standard deviation, SD) unless otherwise indicated. N = number of eyes.
| Central Visual Field Defect (+) (N=408) | Central Visual Field Defect (−) (N=419) | P-value* | |
|---|---|---|---|
| Age (years) | 62.9 (12) | 60 (12) | <0.001 |
| Baseline 24-2 Mean Deviation (dB) | −9.0 (6) | −2.2 (2) | <0.001 |
| Baseline 24-2 Mean Sensitivity Deviation (dB) | −9.0 (6) | −2.5 (2) | <0.001 |
| Baseline 24-2 Visual Field Index (%) | 75.8 (20) | 95.5 (3) | <0.001 |
| Number of Visual Field Tests | 13.7 (6) | 13.7 (6) | 0.978 |
| Follow-up Time (years) | 8.8 (3) | 8.8 (4) | 0.979 |
| Central Corneal Thickness (microns) | 532.8 (39) | 534.4 (39) | 0.554 |
| Mean Intraocular Pressure (mmHg) | 14.5 (3) | 15.8 (3) | <0.001 |
Independent t-test with unequal variances
The mean rate of MD, MSD, and VFI change of the entire sample was −0.15 dB/yr (95% CI: −0.19 to −0.12, P<0.001), −0.15 dB/yr (95% CI: −0.18 to −0.11, P<0.001), and −0.55%/yr (95% CI: −0.65 to −0.44, P<0.001), respectively. Eyes with baseline central damage had greater average MD rate of progression than those without (difference, βcentral: −0.07 dB/year, 95% CI=−0.11 to −0.01, P=0.011). This significant difference in MD rate of progression was also found in those with and without baseline nasal damage (βnasal= −0.05 dB/year, 95% CI= −0.11 to −0.00, P=0.049).
A similar a number of eyes had central or nasal defects at baseline (408 vs. 426, respectively). In addition, there was considerable overlap in the proportion of eyes with simultaneous central and nasal defects (320 of 827 [38%], Table 2).
TABLE 2.
Proportion of sample (number of eyes) with central versus nasal defects at baseline.
| Central Defect | ||||
|---|---|---|---|---|
| No | Yes | Total | ||
| Nasal Defect | No | 313 | 88 | 401 |
| Yes | 106 | 320 | 426 | |
| Total | 419 | 408 | 827 | |
In the multivariable analysis using mixed effects modeling, eyes with baseline central defects progressed faster than those without after adjusting for covariates (difference, βcentral = −0.08 dB/yr, 95% CI: −0.15 to −0.02, P=0.008). Those with nasal defects also progressed faster (βnasal = −0.07 dB/yr, 95% CI: −0.14 to −0.01, P= 0.021). However, when we added central defects and nasal defects together in the same multivariable model, only those with central defects remained significant (P=0.030 vs. 0.090) (Table 3). Similar results were seen when looking at MSD (P= 0.022 vs. 0.124) and VFI rates of progression (P= 0.007 vs. 0.040).
TABLE 3.
Multivariable analysis of the rate of mean deviation (MD, in dB) change over time (in years).
| Variable | β-coefficient | 95% Confidence Interval | P-value |
|---|---|---|---|
| Time 24-2 (years) | 0.68 | 0.32 to 1.04 | <0.001 |
| Baseline Central Visual Field Defect | −0.33 | −0.57 to −0.08 | 0.007 |
| Baseline Central Visual Field *Time | −0.07 | −0.140 −0.006 | 0.030 |
| Baseline Nasal Defect | −0.06 | −0.30 to 0.18 | 0.610 |
| Baseline Nasal Defect *Time | −0.06 | −0.129 to 0.009 | 0.090 |
| Baseline Mean Deviation (per dB) | 0.94 | 0.919 to 0.963 | <0.001 |
| Baseline Mean Deviation*Time | −0.001 | −0.007 to 0.004 | 0.675 |
| Intraocular Pressure (per mmHg) | 0.02 | −0.002 to 0.056 | 0.068 |
| Intraocular Pressure*Time | −0.018 | −0.027 to −0.009 | <0.001 |
| Central Corneal Thickness (per 40 microns) | 0.039 | −0.056 to 0.136 | 0.430 |
| Central Corneal Thickness*Time | 0.004 | −0.015 to 0.026 | 0.600 |
| Age (per decade) | 0.038 | −0.046 to 0.12 | 0.377 |
| Age*Time | −0.095 | −0.124 to −0.066 | <0.001 |
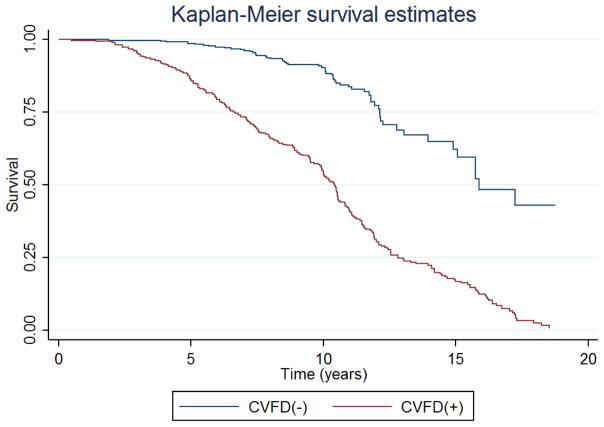
In the multivariable model with event analysis, the hazard ratio for MD loss >5 dB in eyes with baseline central damage was 3.0 (95% CI: 2.1 to 4.1, P<0.001). (Table 4 and Figure 2)
TABLE 4.
Results of the multivariable survival analysis of a > 5 dB MD change from baseline.
| Hazard Ratio | 95% Confidence Interval | P-value | |
|---|---|---|---|
| Baseline Central Field Defect | 3.00 | 2.18 to 4.14 | <0.001 |
| Mean Intraocular Pressure (per mmHg) | 1.03 | 1.005 to 1.06 | 0.021 |
| Central Corneal Thickness (per 40 μm) | 1.04 | 0.92 to 1.17 | 0.460 |
| Age (per decade) | 1.35 | 1.22 to 1.50 | <0.001 |
| Baseline Mean Deviation (per dB) | 0.93 | 0.92 to 0.95 | <0.001 |
FIGURE 2.
Cox proportional hazards survival analysis comparing the probability of progression (> 5 dB change from baseline) between eyes with and without baseline central visual field damage (CVFD).
DISCUSSION
This prospective cohort study demonstrates that baseline central VF loss as measured with the 24-2 VF testing strategy was predictive of more rapid and significant progression of global field damage in eyes with manifest glaucoma. Our findings may have significant clinical implications for closer surveillance as well as more aggressive treatment of glaucoma patients with central VF loss. To our knowledge, the present analysis is the first to prospectively study the effects of central VF damage on global field progression over time in patients with manifest glaucoma.
The main goal of glaucoma therapy is to preserve visual function and quality of life. We found that the presence of baseline central 24-2 damage increases the likelihood of experiencing MD loss > 5 dB (an amount of change known to significantly affect quality-of-life13) by three-fold compared to those without baseline central damage. The strong association between baseline central visual damage and progression of global VF damage demonstrates that these eyes are at greater risk of more rapid global progression. Furthermore, Abe et al recently demonstrated greater declines in NEI VFQ-25 scores in those patients who had worse central VF sensitivity at baseline.9
We employed several strategies to overcome the potential bias of trying to analyze the effect of central VF damage on VF progression as central and global VF damage are strongly correlated. First, as a stronger weighting of central field points on global indices could bias our results, we also assessed rates of MSD change. We also found statistically significant effects in both MD and MSD. These differences between the various global indices should be taken into consideration in future studies assessing VF outcomes. Second, we also tried to minimize the effect of differences in baseline MD as a confounder on MD rates of change by performing a multivariable analysis that adjusts for baseline MD and differences in slopes between eyes with more versus less severe MD (represented by the variable “Baseline MD” and its interaction “Baseline MD*Time”, respectively). Finally, we investigated whether eyes with nasal field defects (meeting the same criteria of abnormality as those with central defects) progressed faster than those without nasal defects. Our rationale for this analysis was that the location of the defect – and not only the overall severity of the VF defect predicts future progression. This type of analysis is also important as many eyes have both nasal and central defects (Table 2). Even though eyes with nasal defects progressed faster than those without it, when both groups (nasal and central) were entered together in the same model, only central defects remained significant suggesting not only the importance of VF severity but, more importantly, the location of the defect. The results of all the analyses described above provide consistent evidence that baseline central damage predicts global VF MD progression independent of worse overall VF damage found in patients with central loss.
Progression of global VF loss associated with baseline central damage has also been evaluated in a retrospective study of normal tension glaucoma by Membrey and colleauges.4 In this study, central field loss was defined as presence of a cluster of at least three points in one hemifield depressed by −5 dB, with at least one point depressed by >10 dB in the four paracentral locations. Progression was defined as −1 dB per year or more of sensitivity loss at the same test location. The authors found that eyes with baseline threats to fixation tended to progress more rapidly globally although this was not statistically significant (P=0.18).4 Additionally, in a retrospective study with normal tension glaucoma patients, Cho et al compared the rates of sensitivity change between eyes with initial paracentral defects and initial peripheral defects. No significant differences were found in testing sectoral and global rates of change between the two groups.19 Furthermore, Nassiri et al used pointwise event analysis to study glaucomatous eyes with a mean baseline MD of −4.2 ± 4.5 dB and found that rates of decay were faster in the central 10° surrounding fixation compared to 20° and 30° regions.20 Differences in study design, samples, and definitions of central VF and progression may explain at least in part inconsistencies between our findings and those cited above.
Other reports have explored patterns of damage in the VF and their association with more rapid progression. Shon et al proposed that superior arcuate as well as superior and inferior nasal areas may be best able to predict future VF deterioration as these regions demonstrated the most consistent rate of reduction in retinal sensitivity. In this study, reduction of central sensitivity was found to be variable between early and late follow-up period.21 While this report offers useful insight into VF progression, we cannot definitively conclude that a consistent rate of progression in any given area makes it a solid predictor for overall progression. Additionally, this study used 24-2 VFs to study the macular region; inconsistent results in this region may be due to variable sampling of the damage in this region.
Our study found that central VF defects are linked to a faster decline in global progression of the field. It is known that decline in visual function has a significant effect on quality of life.13, 22–24 Abe et al reported that reduction of sensitivity in the central inferior region of the 24-2 VF was found to have the highest association with decline in vision-related quality of life.9 This association between central damage, decline in quality of life, and faster global progression can have significant ramifications for patients and requires physicians to pay special attention to changes in the central VF. Furthermore, the vulnerability of the central field to elevated pressures25 calls for a more aggressive treatment regimen in the setting of central loss.
Our analysis used only the 24-2 strategy to study the central VF; however previous studies have found that central visual damage can be missed with 24-2 fields.26 Despite this potential disadvantage of 24-2 tests to detect central VF loss, clinicians more commonly use 24-2 over 10-2 testing to monitor progression in glaucoma patients. Therefore, our findings using 24-2 fields have more direct clinical applicability. Nonetheless, our results need to be further validated with a long term study using both 24-2 and 10-2 testing strategies. Additionally, proposed reasons for macular loss in glaucoma include nasalization of the central retinal vessel trunk,27 axonal crowding at the inferior and superior poles of the nerve,28 and the enlargement of lamina cribrosa pores and associated loss of structural support in these regions.29 Further investigations to corroborate these anatomical changes with functional testing would be useful. Of note, our results suggest an association between central functional loss and rates of global visual field progression. A causal relationship cannot be inferred from our results and future studies ought to address this matter
In conclusion, the presence of central VF damage is significantly associated with increased velocity of global VF progression. Detection of central VF damage aids in stratification of high-risk patients who may need more intensive surveillance and aggressive treatment.
Supplementary Material
Acknowledgments
-
Funding/Support:
National Eye Institute Grants: U10EY14267, EY08208, EY11008, EY019869, EY13959, 1EY027510; EY025253 (CGDM); Eyesight Foundation of Alabama; Alcon Laboratories Inc.; Allergan Inc.; Pfizer Inc.; Merck Inc.; Santen Inc.; unrestricted departmental grant from Research to Prevent Blindness, New York, NY (Department of Ophthalmology, Columbia University Medical Center and Department of Ophthalmology, University of California San Diego), Edith C. Blum Foundation, New York, NY, Bernard Schwartz Travel Grant from the American Glaucoma Society, San Francisco, CA.
-
Financial disclosures:
A.G.: No financial disclosures.
C.G.D.M.: No financial disclosures.
C.A.G.: Research support – Heidelberg Engineering GmbH.
F.A.M.: Financial support – Alcon Laboratories Inc., Carl Zeiss Meditec Inc., Pfizer Inc.; Consultant – Alcon Laboratories Inc., Allergan Inc., Pfizer Inc.; Research support – Alcon Laboratories Inc., Allergan Inc., Carl Zeiss Meditec Inc., Pfizer Inc., Reicherts Inc.
R.N.W.: Financial support – Carl Zeiss Meditec Inc., Heidelberg Engineering GmbH, Optovue Inc., Topcon Medical Systems; Consultant – Aerie, Alcon Laboratories Inc., Allergan Inc, Bausch & Lomb, Unity; Grants – Quark, Genentech.
L.M.Z.: Research support and equipment – Carl Zeiss Meditec Inc., Heidelberg Engineering GmbH, Optovue Inc., Topcon Medical Systems Inc.;
J.M.L.: Consultant – Sensimed, Inc., Carl Zeiss Meditec, Inc., Alcon Laboratories, Topcon, Inc., Heidelberg Engineering, GmBH, Allergan, Inc., Bausch& Lomb, Inc, Aerie Pharmaceuticals, Inc, Quark Pharmaceuticals, Inc.
-
Other Acknowledgements:
None.
Biography

Aakriti Garg MD is currently a third-year ophthalmology resident at Columbia University Medical Center in New York, New York. Dr. Garg obtained her medical degree from Columbia University College of Physicians and Surgeons. Her primary areas of research interest include identifying risk factors for visual field progression, imaging in glaucoma, as well as glaucoma’s impact on vision-related quality of life.
Footnotes
Presented in part at the Annual Meeting of the American Glaucoma Society (AGS), Coronado, CA, March, 2017.
Trial Registration: clinicaltrials.gov Identifier: NCT00221923
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.De Moraes CG, Prata TS, Tello C, Ritch R, Liebmann JM. Glaucoma with early visual field loss affecting both hemifields and the risk of disease progression. Archives of ophthalmology (Chicago, Ill: 1960) 2009;127:1129–34. doi: 10.1001/archophthalmol.2009.165. [DOI] [PubMed] [Google Scholar]
- 3.Cho HK, Kee C. Comparison of the progression rates of the superior, inferior, and both hemifield defects in normal-tension glaucoma patients. Am J Ophthalmol. 2012;154:958–968e1. doi: 10.1016/j.ajo.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Membrey WL, Poinoosawmy DP, Bunce C, Fitzke FW, Hitchings RA. Comparison of visual field progression in patients with normal pressure glaucoma between eyes with and without visual field loss that threatens fixation. Br J Ophthalmol. 2000;84:1154–8. doi: 10.1136/bjo.84.10.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumberg DM, De Moraes CG, Prager AJ, et al. Association Between Undetected 10-2 Visual Field Damage and Vision-Related Quality of Life in Patients With Glaucoma. JAMA Ophthalmol. 2017 doi: 10.1001/jamaophthalmol.2017.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prager AJ, Hood DC, Liebmann JM, et al. Association of Glaucoma-Related, Optical Coherence Tomography-Measured Macular Damage With Vision-Related Quality of Life. JAMA Ophthalmol. 2017 doi: 10.1001/jamaophthalmol.2017.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Lin C, Waisbourd M, et al. The Impact of Visual Field Clusters on Performance-based Measures and Vision-Related Quality of Life in Patients With Glaucoma. Am J Ophthalmol. 2016;163:45–52. doi: 10.1016/j.ajo.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Ekici F, Loh R, Waisbourd M, et al. Relationships Between Measures of the Ability to Perform Vision-Related Activities, Vision-Related Quality of Life, and Clinical Findings in Patients With Glaucoma. JAMA ophthalmology. 2015;133:1377–85. doi: 10.1001/jamaophthalmol.2015.3426. [DOI] [PubMed] [Google Scholar]
- 9.Abe RY, Diniz-Filho A, Costa VP, Gracitelli CP, Baig S, Medeiros FA. The Impact of Location of Progressive Visual Field Loss on Longitudinal Changes in Quality of Life of Patients with Glaucoma. Ophthalmology. 2016;123:552–7. doi: 10.1016/j.ophtha.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita K, Yasuda N, Oda K, Yuzawa M. Reading performance in patients with central visual field disturbance due to glaucoma. Nippon Ganka Gakkai Zasshi. 2006;110:914–8. [PubMed] [Google Scholar]
- 11.Coeckelbergh TR, Cornelissen FW, Brouwer WH, Kooijman AC. The effect of visual field defects on eye movements and practical fitness to drive. Vision Res. 2002;42:669–77. doi: 10.1016/s0042-6989(01)00297-8. [DOI] [PubMed] [Google Scholar]
- 12.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–45. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R Los Angeles Latino Eye Study G. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:941–948e1. doi: 10.1016/j.ophtha.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heijl A. Studies on computerized perimetry. Acta Ophthalmol Suppl. 1977;132:1–42. [PubMed] [Google Scholar]
- 15.LeBlanc EP, Becker B. Peripheral nasal field defects. Am J Ophthalmol. 1971;72:415–9. doi: 10.1016/0002-9394(71)91314-6. [DOI] [PubMed] [Google Scholar]
- 16.Werner EB, Beraskow J. Peripheral nasal field defects in glaucoma. Ophthalmology. 1979;86:1875–8. doi: 10.1016/s0161-6420(79)35335-0. [DOI] [PubMed] [Google Scholar]
- 17.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–7. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 18.Racette L, Wilson MR, Zangwill LM, Weinreb RN, Sample PA. Primary open-angle glaucoma in blacks: a review. Surv Ophthalmol. 2003;48:295–313. doi: 10.1016/s0039-6257(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 19.Cho HK, Lee J, Lee M, Kee C. Initial central scotomas vs peripheral scotomas in normal-tension glaucoma: clinical characteristics and progression rates. Eye (Lond) 2014;28:303–11. doi: 10.1038/eye.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassiri N, Moghimi S, Coleman AL, Law SK, Caprioli J, Nouri-Mahdavi K. Global and pointwise rates of decay in glaucoma eyes deteriorating according to pointwise event analysis. Invest Ophthalmol Vis Sci. 2013;54:1208–13. doi: 10.1167/iovs.12-10833. [DOI] [PubMed] [Google Scholar]
- 21.Shon K, Wollstein G, Schuman JS, Sung KR. Prediction of glaucomatous visual field progression: pointwise analysis. Curr Eye Res. 2014;39:705–10. doi: 10.3109/02713683.2013.867353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prager AJ, Hood DC, Liebmann JM, et al. Association of Glaucoma-Related, Optical Coherence Tomography-Measured Macular Damage With Vision-Related Quality of Life. JAMA Ophthalmol. 2017;135:783–788. doi: 10.1001/jamaophthalmol.2017.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumberg DM, De Moraes CG, Prager AJ, et al. Association Between Undetected 10-2 Visual Field Damage and Vision-Related Quality of Life in Patients With Glaucoma. JAMA Ophthalmol. 2017;135:742–747. doi: 10.1001/jamaophthalmol.2017.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP Los Angeles Latino Eye Study G. Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143:1013–2. doi: 10.1016/j.ajo.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolker AE. Visual prognosis in advanced glaucoma: a comparison of medical and surgical therapy for retention of vision in 101 eyes with advanced glaucoma. Trans Am Ophthalmol Soc. 1977;75:539–55. [PMC free article] [PubMed] [Google Scholar]
- 26.Grillo LM, Wang DL, Ramachandran R, et al. The 24-2 Visual Field Test Misses Central Macular Damage Confirmed by the 10-2 Visual Field Test and Optical Coherence Tomography. Transl Vis Sci Technol. 2016;5:15. doi: 10.1167/tvst.5.2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Wang H, Pasquale LR, et al. Relationship Between Central Retinal Vessel Trunk Location and Visual Field Loss in Glaucoma. Am J Ophthalmol. 2017;176:53–60. doi: 10.1016/j.ajo.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hood DC, Raza AS, de Moraes CG, Johnson CA, Liebmann JM, Ritch R. The Nature of Macular Damage in Glaucoma as Revealed by Averaging Optical Coherence Tomography Data. Transl Vis Sci Technol. 2012;1:3. doi: 10.1167/tvst.1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981;99:137–43. doi: 10.1001/archopht.1981.03930010139020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.