Figure 2.
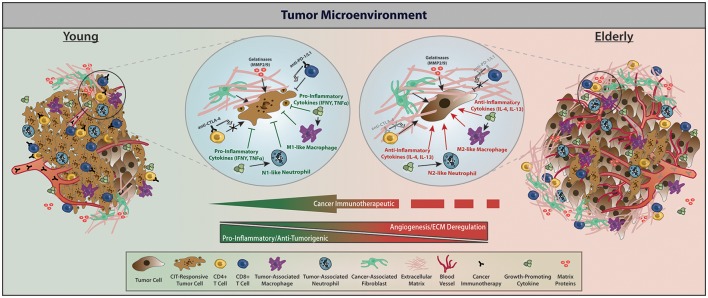
With respect to factors affecting CIT, the TME of young vs. elderly hosts differs predominately in immune infiltration and cytokine profile, regulation of angiogenesis and the ECM, as well as interactions between tumor and stromal cells. Within the young TME, increased presence of CIT-responsive tumor cells, less angiogenesis and ECM deregulation, elevated immune infiltration, and elevated pro-inflammatory cytokines give rise to a relatively more CIT-responsive TME, subsequently contributing to apoptotic tumor cells with reduced proliferation. Within elderly TMEs, relatively bigger tumors but with fewer CIT-responsive tumor cells, more angiogenesis and ECM deregulation, decreased immune infiltration, and elevated anti-inflammatory cytokines contribute to reduced apoptosis and increased proliferation subsequently enabling tumor growth. Treatment with CIT in elderly individuals triggers a phenotypic landscape remodeling toward a TME with young characteristics through enhancing the function of effector T cells following CIT and promoting a pro-inflammatory TME. Within elderly hosts, the tumor stroma permits a deregulated ECM that creates a biophysical barrier preventing effective function of effector T cells, but CIT combinations may help alleviate these age-related TME dysfunctions and decrease tumor burden. Of note—while we acknowledge that there are dormant and/or tumor initiating cells, we only depict CIT responders vs. non-responders within the TME (Gonzalez et al., 2017).