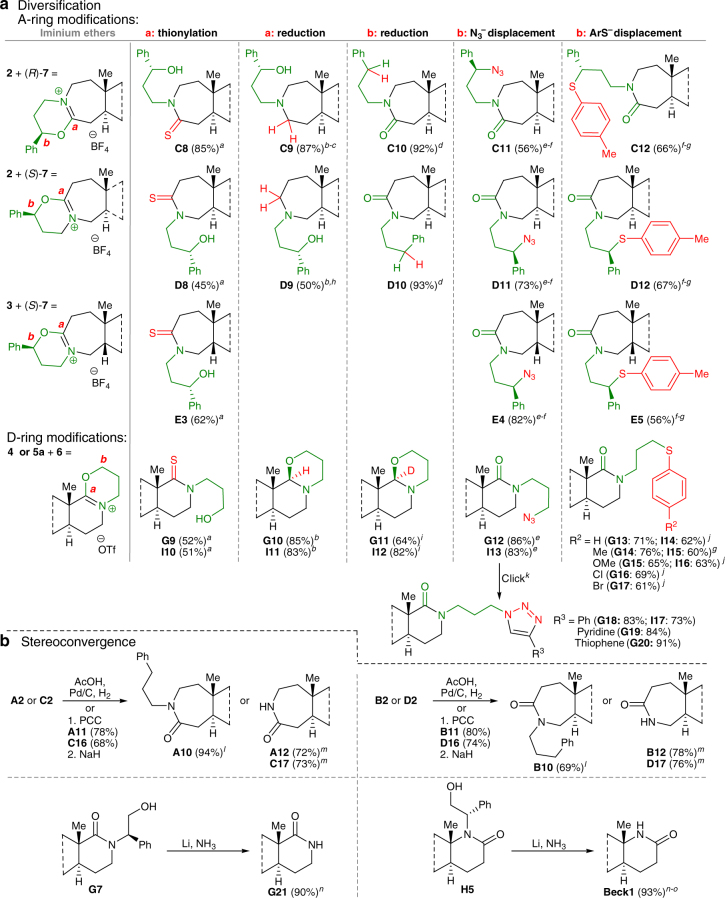
Fig. 4.
Structural diversification. a Diversification of iminium ethers for A-ring and D-ring iminium ether intermediates. Green text highlights the azide component and red text highlights the nucleophile component; positions a and b (in red) mark the sites of particular transformations. Yields refer to isolated yields of products after chromatography on silica. b Stereoconvergence and synthesis of parent NH lactams. Yields refer to isolated yields of products after chromatography on silica. aNa2S, THF or DMF, 65 °C. bNaBH4, MeOH, rt. cC9 was also obtained following LAH reduction of C2 (51%). dHydrogenation using 10% Pd/C, EtOH. eNaN3, DMF, 70 °C. fInversion of stereochemistry. g4-Methylbenzenethiol, DMF, 75 °C. hD9 was also obtained following LAH reduction of D2 (58%). iNaBD4, MeOH, rt. jStock solutions of sodium thiophenoxides, DMF, 75 °C. kSubstituted acetylene, CuSO4•5H2O, sodium L-ascorbate, tBuOH/H2O. lHydrogenation using 10% Pd/C, AcOH, EtOH. mRemoval of non-benzylic side chain via treatment with base. nRemoval of benzylic side chain via dissolving metal reduction. oIsolated product is Beck1. See Supplementary Methods for full synthetic details. C and D are 5α-dihydrotestosterone derivatives; E and F are 5β-dihydrotestosterone derivatives; G are trans-androsterone derivatives; I are estrone derivatives. AcOH, acetic acid; PCC, pyridinium chlorochromate; NaH, sodium hydride in 60% mineral oil; Na2S, sodium sulfide; THF, tetrahydrofuran; DMF, dimethylformamide; NaBH4, sodium borohydride; MeOH, methanol; EtOH, ethanol, LAH, lithium aluminum hydride in 1 M THF; NaN3, sodium azide; NaBD4, sodium borodeuteride; tBuOH, tert-butanol; H2O, distilled water