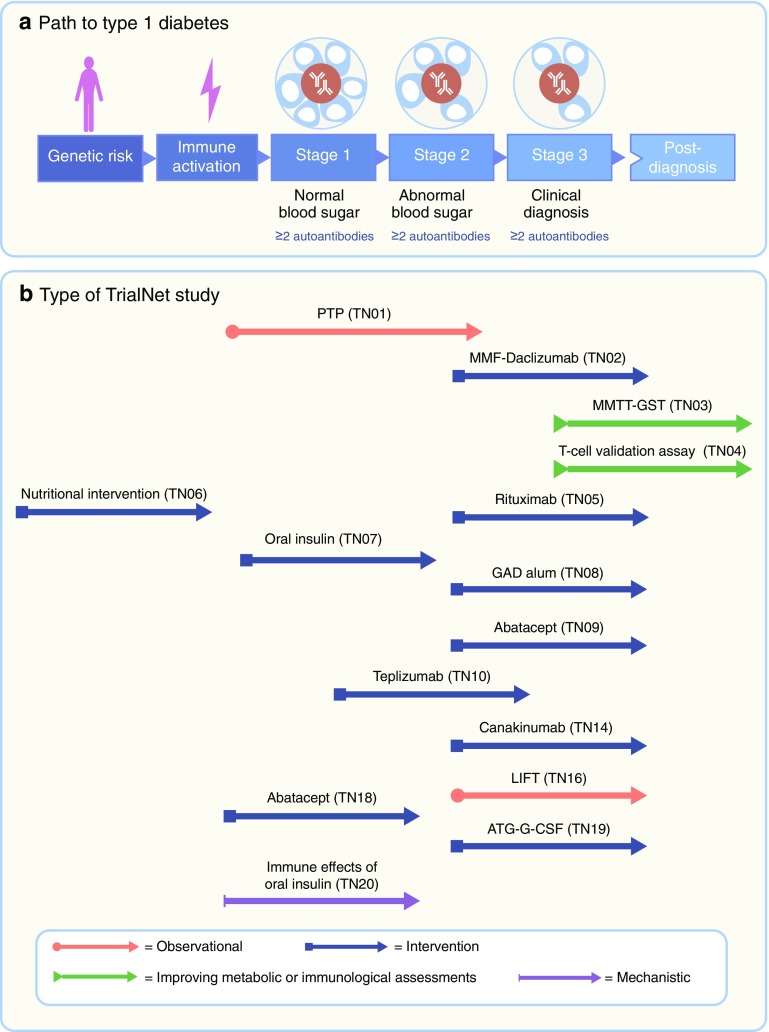
Fig. 1.
Type 1 diabetes: a disease with three stages of pathology and various opportunities for intervention with disease-modifying therapies and mechanistic analysis. (a) Stage 1 is the start of the disease—there are no symptoms and blood sugar remains normal but the autoimmune process has already begun, manifested by multiple autoantibodies against beta cell targets. In stage 2, like stage 1, autoimmunity is the key feature and there are no symptoms; however, blood sugar control has now become abnormal due to loss of beta cells. From stages 1 and 2, there is an extremely high risk of progression to stage 3, when symptoms of diabetes emerge (thirst, weight loss and fatigue) due to significant beta cell loss, and the clinical diagnosis is made. (b) Each stage of the disease is encompassed within TrialNet and offers an opportunity for interventions or mechanistic analysis. Examples of TrialNet studies (concluded and ongoing) are listed in association with the targeted disease stage. ATG-G-CSF, anti-thymocyte globulin+granulocyte colony stimulating factor; GST, glucagon stimulation test; LIFT, long-term investigative follow-up; MMF, mycophenolate mofetil; MMTT, mixed meal tolerance test; PTP, Pathway to Prevention study. Abatacept is a CTLA-4–immunoglobulin; canakinumab is an anti-IL1β monoclonal antibody (mAb); rituximab is an anti-CD20 mAb; teplizumab is an anti-CD3 mAb